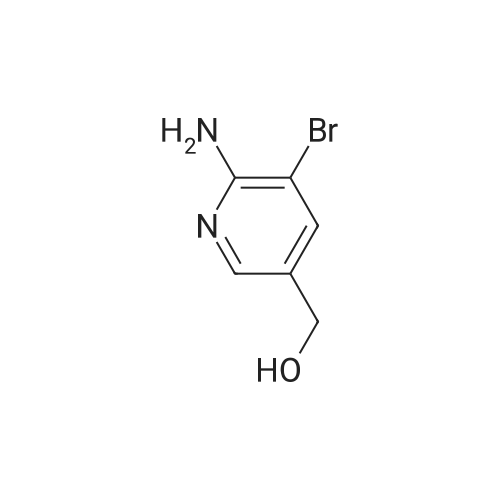
| 61% |
With N-chloro-succinimide; In N,N-dimethyl-formamide; at -20 - 20℃;Inert atmosphere; |
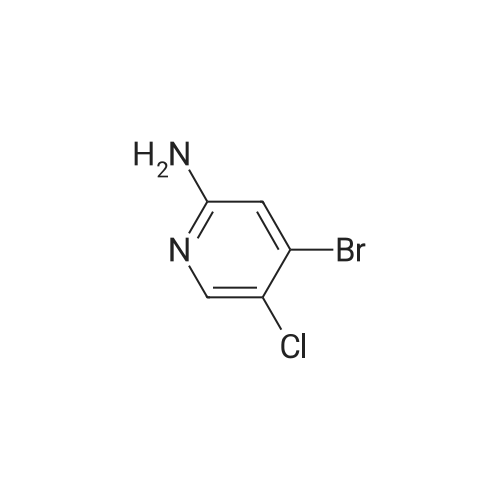
Description 14 (D14); 4-bromo-5-chloro-2-pyridinamine; To a stirred solution of 2-amino-4-bromopyridine (15.0 g, 86.7 mmol) in N, N- dimethylformamide (430 ml_) at -200C was added N-chlorosuccinimide (12.99 g, 95.34 mmol). Reaction mixture allowed to stir at room temperature for 24 h. Reaction mixture poured into cold 1 M sodium hydroxide (750 ml_) and extracted with diethyl ether (4 x 500 ml_). The combined extracts were washed with water (3 x 200 ml_), brine (200 ml_), dried over sodium sulfate, filtered and solvent removed in vacuo yielding a solid (17.2 g) which was recrystallised from hexane to yield a solid (12.8 g). Material purified by column <n="70"/>chromatography eluting with 0-25% ethyl acetate in dichloromethane. The relevant fractions were combined and solvent removed in vacuo to furnish the title compound (1 1.0 g, 61 %). 1H NMR: (300 MHz, CDCI3) delta 8.05 (s, 1 H), 6.79 (s, 1 H), 4.49 (s, 2H). |
| 43.7% |
With N-chloro-succinimide; In N,N-dimethyl-formamide; at -78 - 20℃;Inert atmosphere; |
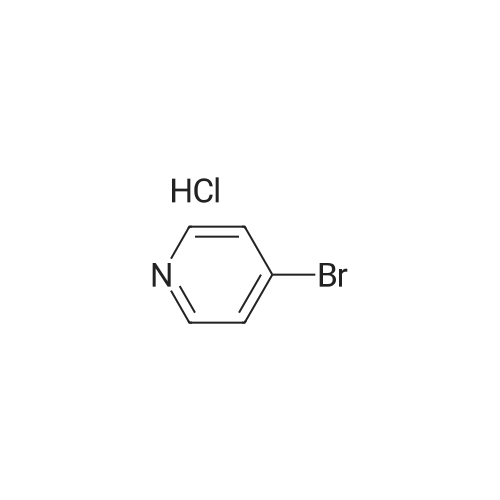
N-Chloro-succinimide (3.70 g, 27.7 mmol) dissolved in DMF (20 mL) was added dropwise to 4-bromopyridin-2-amine (4.40 g, 25.4 mmol) in DMF (50 mL) at -78 C. over a period of 30 minutes under nitrogen. The resulting suspension was then allowed to warm to r.t. After stirring under these conditions for 24 h, the reaction mixture was diluted with Et2O (50 mL) and washed sequentially with 1 M aqueous NaOH (2×50 mL), water (50 mL), and saturated aqueous sodium chloride (25 mL). The organic layer was dried over MgSO4, filtered and concentrated under reduced pressure. The resulting crude product was purified by flash silica chromatography, elution gradient 0 to 25% EtOAc in DCM. Pure fractions were evaporated to dryness to afford 4-bromo-5-chloropyridin-2-amine (2.30 g, 43.7%) as a cream solid. 1H NMR (400 MHz, DMSO-d6, 30 C.) 6.35 (2H, s), 6.82 (1H, s), 8.01 (1H, s). m/z: ES+[M+H]+ 209 (35C1 81Br and 37C1 79Br isotopes). |
|
With N-chloro-succinimide; In N,N-dimethyl-formamide; at 20℃; for 12h; |
To a solution of 4-bromopyridin-2-amine (500 mg, 2.89 mmol) in N,N-dimethylformamide (5 mL) was added N-chlorosuccinimide (463 mg, 3.47 mmol) and the mixture was stirred at room temperature for 12 hours. The mixture was filtered through diatomaceous earth and concentrated and the residue was dissolved in ethyl acetate. The solution was washed with water and brine, dried over anhydrous sodium sulfate, filtered, and concentrated to afford the crude title compound. Purification by column chromatography (silica gel, 30 % ethyl acetate in hexane) afforded the title compound. |
|
With N-chloro-succinimide; In N,N-dimethyl-formamide; at -20 - 20℃; for 16h; |
To a solution of 4-bromopyridin-2-amine (12.0 g, 69.4 mmol) in N,N-dimethylformamide (200 mL) at -20 C. was slowly added a solution of 1-chloropyrrolidine-2,5-dione (10.24 g, 77.0 mmol) in N,N-dimethylformamide (200 mL). The mixture was stirred at room temperature for 16 hours and the mixture was poured into cold 1M aqueous sodium hydroxide (1000 mL) and extracted with ethyl acetate. The organic layer was washed with water and brine, dried over sodium sulfate, filtered and concentrated. Purification by column chromatography on silica (Analogix 280), eluting with a gradient of 5-65% ethyl acetate/hexanes gave the title compound. MS (ESI) m/e 208 (M+H)+. |
|
With N-chloro-succinimide; In N,N-dimethyl-formamide; at -20 - 20℃; for 24h; |
General procedure: 5.2.2.30 7,8-Dichloro-1,4-dihydropyrido[2,3-b]pyrazine-2,3-dione (30) To a solution of 2-amino-4-chloropyridine (1.28 g, 10.0 mmol) in DMF (40 mL) at -20 C was added NCS (2.67 g, 20.0 mmol). This mixture was allowed to warm to room temperature and stirred for 24 h, and then poured into 300 mL ice-water and extracted with ethyl acetate. The extracts were washed with 1 M NaOH and brine, dried and evaporated. The residue was purified by column chromatography on silica gel to give 4,5-dichloropyridin-2-amine (1.12 g, 69.0%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping