| 39% |
With N-ethyl-N,N-diisopropylamine; In acetonitrile; at 130℃; for 21h;Microwave irradiation; |
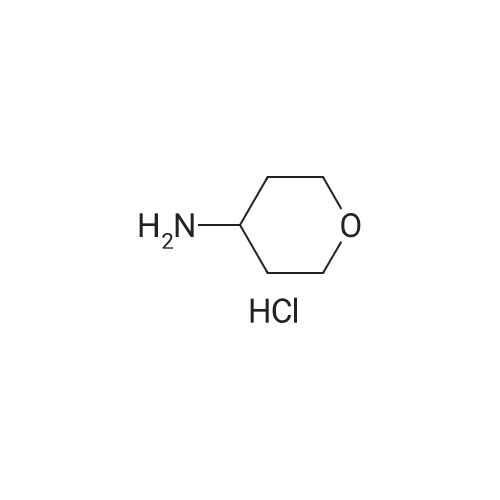
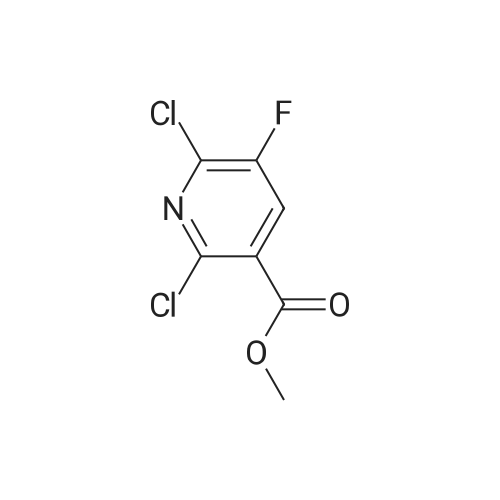
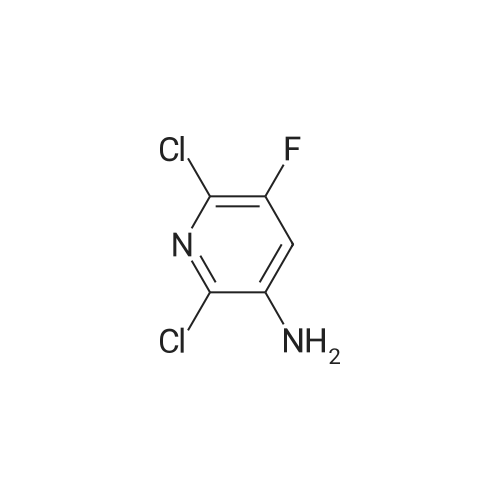
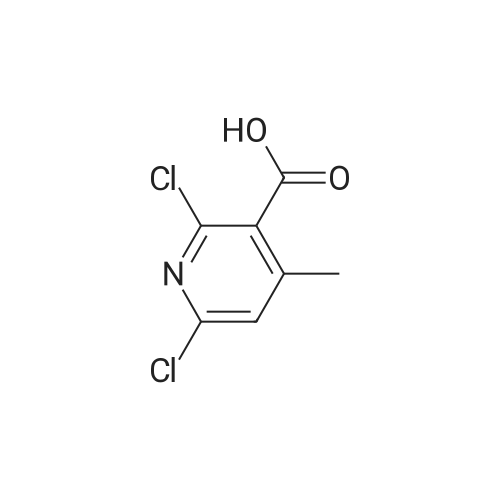
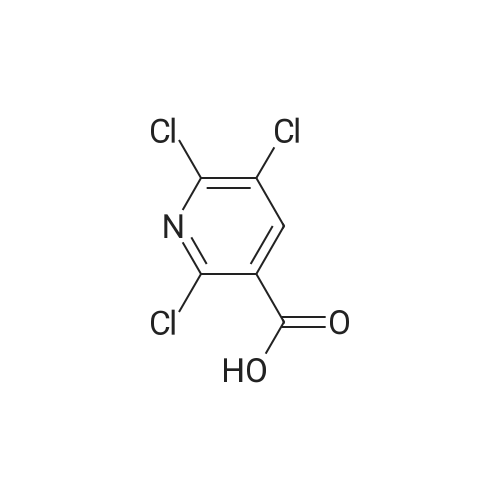
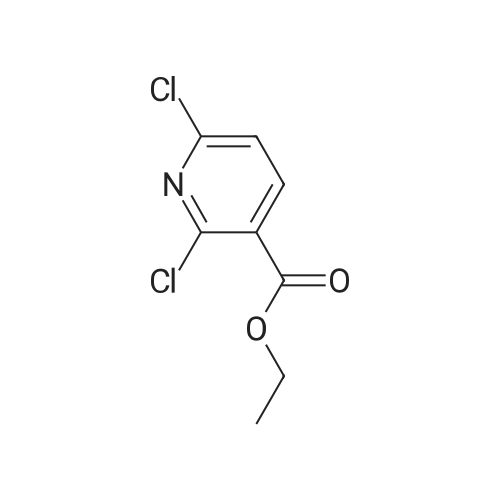
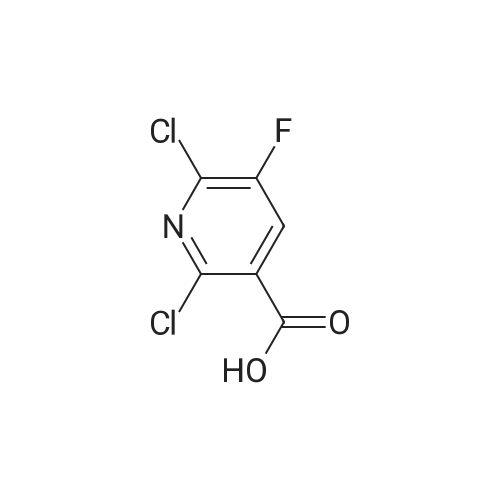
PREPARATION 79 5-Chloro-6-fluoro-3-(tetrahydro-2H-pyran-4-yl)-1-[2-(trimethylsilyl)ethoxy] methyl}-1,3-dihydro-2H-imidazo[4,5-b]pyridin-2-one [Show Image]a) 6-Chloro-5-fluoro-2-(tetrahydro-2H-pyran-4-ylamino)nicotinic acid A mixture of 2,6-dichloro-5-fluoronicotinic acid (1.01 g, 4.8 mmol), diisopropylethylamine (11 mL, 62.5 mmol) and <strong>[33024-60-1]tetrahydro-2H-pyran-4-amine hydrochloride</strong> (3.30 g, 24 mmol) in acetonitrile (5 mL) was stirred and heated under microwave irradiation ("Initiator sixty" from Biotage.(R).) at 130 °C for 21 hours. The mixture was then cooled and dichloromethane was added and the organic layer was washed with 5percent aqueous citric acid, water, brine, dried (MgSO4) and the solvent was evaporated. The residue was purified by reverse phase chromatography (C-18 silica from Waters.(C)., water/acetonitrile/methanol as eluents [0.1percent v/v formic acid buffered] 0percent to 100percent) to give the title compound (0.51 g, 39percent) of as a white solid. LRMS (m/z): 273 (M-1)+.1H NMR (300 MHz, CDCl3) delta ppm 1.48 - 1.69 (m, 2H), 1.97 - 2.14 (m, 2H), 3.58 (t, 2H), 4.03 (dd, 2H), 4.29 (ddd, 1H), 5.04 (d, 1H), 7.84 (d, 1H). |
| 39% |
With N-ethyl-N,N-diisopropylamine; In acetonitrile; at 130℃; for 21h;Microwave irradiation; |
PREPARATION 79 5-Chloro-6-fluoro-3-(tetrahydro-2H-pyran-4-yl)-1-[2-(trimethylsilyl)ethoxy] methyl}-1,3-dihydro-2H-imidazo[4,5-b]pyridin-2-one a) 6-Chloro-5-fluoro-2-(tetrahydro-2H-pyran-4-ylamino)nicotinic acid A mixture of 2,6-dichloro-5-fluoronicotinic acid (1.01 g, 4.8 mmol), diisopropylethylamine (11 mL, 62.5 mmol) and <strong>[33024-60-1]tetrahydro-2H-pyran-4-amine hydrochloride</strong> (3.30 g, 24 mmol) in acetonitrile (5 mL) was stirred and heated under microwave irradiation ("Initiator sixty" from Biotage.(R).) at 130 °C for 21 hours. The mixture was then cooled and dichloromethane was added and the organic layer was washed with 5percent aqueous citric acid, water, brine, dried (MgSO4) and the solvent was evaporated. The residue was purified by reverse phase chromatography (C-18 silica from Waters.(C)., water/acetonitrile/methanol as eluents [0.1percent v/v formic acid buffered] 0percent to 100percent) to give the title compound (0.51 g, 39percent) of as a white solid. LRMS (m/z): 273 (M-1)+.1H NMR (300 MHz, CDCl3) delta ppm 1.48 - 1.69 (m, 2H), 1.97 - 2.14 (m, 2H), 3.58 (t, 2H), 4.03 (dd, 2H), 4.29 (ddd, 1H), 5.04 (d, 1H), 7.84 (d, 1H). |
| 39% |
|
a)6-Chloro-5-fluoro-2-(tetrahydro-2H-pyran-4-ylamino)nicotinic acidA mixture of 2,6-dichloro-5-fluoronicotinic acid (1.01 g, 4.8 mmol), diisopropylethylamine (11 mL, 62.5 mmol) and <strong>[33024-60-1]tetrahydro-2H-pyran-4-amine hydrochloride</strong> (prepared as described in ), 3.30 g, 24 mmol) in acetonitrile (5 mL) was stirred and heated under microwave irradiation at 130 °C for 21 hours.The mixture was then cooled, dichloromethane was added and the organic layer was washed with 5percent aqueous citric acid solution, water and brine, dried (MgSO4) and the solvent was evaporated.The residue was purified by reverse phase chromatography (C-18 silica from Waters?, water/acetonitrile/methanol as eluents [0.1percent v/v formic acid buffered] 0percent to 100percent) to give the title compound (0.51 g, 39percent) as a white solid.LRMS (m/z): 273 (M-1)+.1H NMR delta (300 MHz, CDCl3): 1.48-1.69 (m, 2H), 1.97-2.14 (m, 2H), 3.58 (t, 2H), 4.03 (dd, 2H), 4.29 (ddd, 1H), 5.04 (d, 1H), 7.84 (d, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping