Alternatived Products of [ 822-55-9 ]
Product Details of [ 822-55-9 ]
| CAS No. : | 822-55-9 |
MDL No. : | MFCD00266718 |
| Formula : |
C4H6N2O
|
Boiling Point : |
No data available |
| Linear Structure Formula : | C3H3N2(CH2OH) |
InChI Key : | QDYTUZCWBJRHKK-UHFFFAOYSA-N |
| M.W : |
98.10
|
Pubchem ID : | 1745 |
| Synonyms : |
|
Application In Synthesis of [ 822-55-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 822-55-9 ]
- 1
-
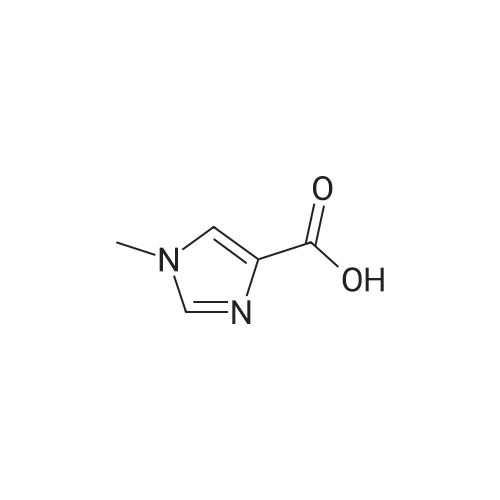
 [ 822-55-9 ]
[ 822-55-9 ]

-
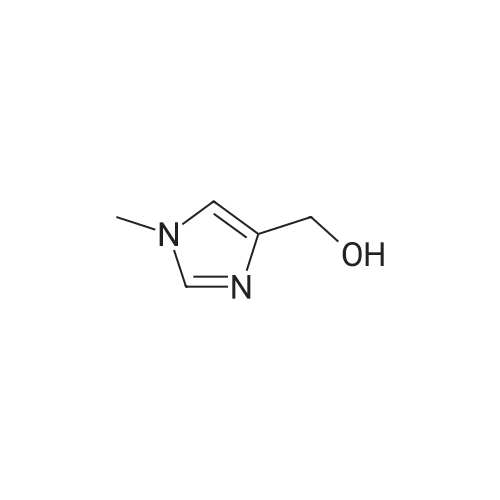 [ 17289-25-7 ]
[ 17289-25-7 ]

-
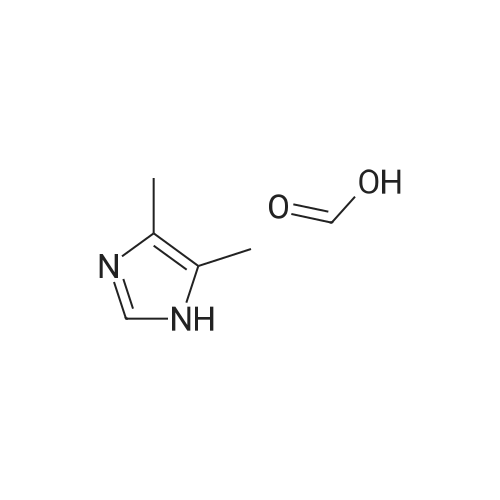
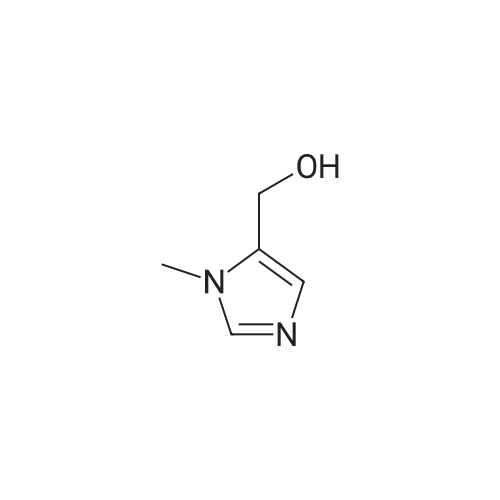 [ 38993-84-9 ]
[ 38993-84-9 ]
- 2
-
 [ 822-55-9 ]
[ 822-55-9 ]

-
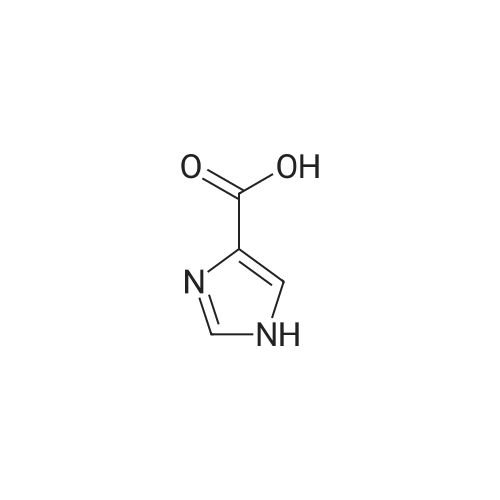 [ 1072-84-0 ]
[ 1072-84-0 ]
- 3
-
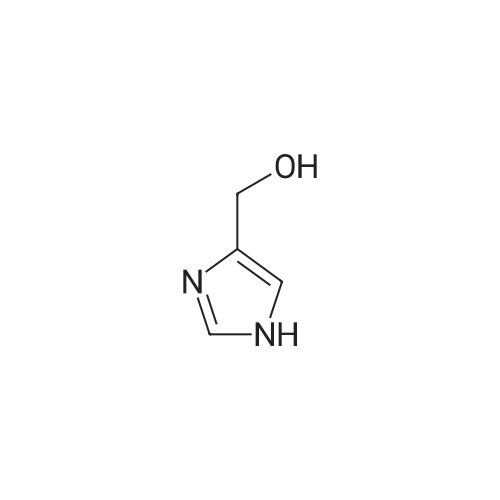
 [ 1072-84-0 ]
[ 1072-84-0 ]

-
 [ 822-55-9 ]
[ 822-55-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In sulfuric acid; |
EXAMPLE 2 A solution of 4-imidazolecarboxylic acid (0.56 g) in 25percent v/v sulphuric acid (25 cc) was placed in the cathodic compartment of a vessel as described in example 1, and 25percent v/v sulphuric acid was placed in the anodic compartment. The mixture was electrolysed for 31/2 hours under conditions similar to those described in Example 1. The solution from the cathodic compartment was neutralised with potassium carbonate (18.5 g) and the mixture was evaporated to dryness. The solid residue was extracted with hot isopropanol (200 cc) and the extract was evaporated to an oil which was crystallized from ether to give 4-(hydroxymethyl)imidazole (0.42 g) m.p. 66°-80°. The picrate derivative of this material was recrystallized from water and had m.p. 205.5°. |
- 4
-
 [ 822-55-9 ]
[ 822-55-9 ]

-
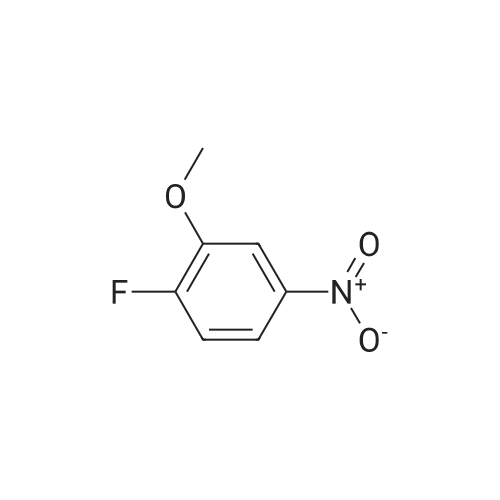 [ 454-16-0 ]
[ 454-16-0 ]

-
 [ 1077629-45-8 ]
[ 1077629-45-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With caesium carbonate; In acetonitrile;Reflux; |
Example 27(l-{4-[4-(2-Chloro-pyridin-3-yloxy)-6-methoxy-[l,3,5]triazin-2-ylamino]-2-methoxy- phenyl}-lH-imidazol-4-yl)-methanola) [ 1 -(2-Methoxy-4-nitro-phenyl)- 1 H-imidazo 1-4-yl] -methano 1A mixture of l-fluoro-2-methoxy-4-nitro-benzene (1.0 g, 5.8 mmol), (1 H-imidazo 1-4-yl)- methanol (602 mg, 6.1 mmol) and cesium carbonate (2.86 g, 8.8 mmol) in 40 ml of acetonitrile was refluxed overnight. The reaction mixture was concentrated in vacuo, diluted with water and extracted with ethyl acetate. Chromatography on Si-NH2 (Isolute) using ethyl acetate as an eluent gave the title compound as a yellowish solid.MS ISP (m/e): 250.1 (51) [(M+H)+]1H NMR (CDCl3, 300 MHz): delta (ppm) = 8.00-7.85 (m, 3H), 7.45 (d, 1 H), 7.26 (d, IH), 4.70 (s, 2H), 4.01 (s, 3H). |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 100℃; for 16h; |
Example A37 a) Preparation of intermediate 98 A mixture of l-fluoro-2-methoxy-4-nitrobenzene (2.45 g, 14.3 mmol), 4- hydroxymethyl- IH- imidazole (1.54 g, 15.7 mmol) and K2CO3 (3.95 g, 28.6 mmol) in DMF (20 ml) was stirred at 100 0C for 16 h. The mixture was concentrated in vacuo, and the residue was partitioned between EtOAc and water. Undissolved material was collected by filtration and dissolved in a mixture of TEtaF and CEta3CN. The combined organic layers were dried (MgSO4), filtered and the solvent was evaporated. The residue was triturated in DIPE/2-propanol, filtered off and dried. Yield: 1.2 g of intermediate 98 which was used as such in the next reaction step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping