|
Sodium phenylphosphinate; In 1,2-dichloro-benzene; at 180 - 230℃; for 7h;Product distribution / selectivity; |
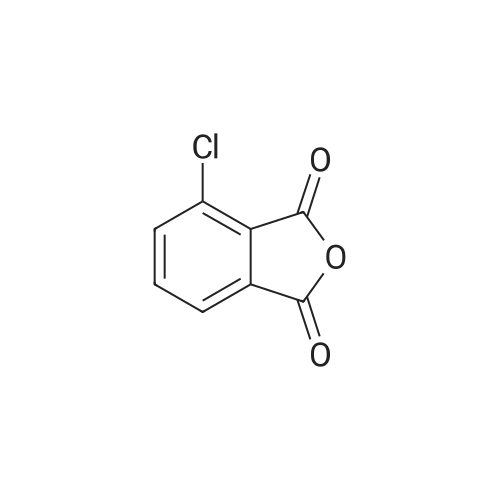
Comparative Example-2; Imidization in Parr Reactor with Up-Front CLPA Addition; A 450 ml Parr pressure-rated reactor equipped as in Comparative Example-1 with two side quartz windows and a partial condenser was charged with solid <strong>[117-21-5]3-chlorophthalic anhydride</strong> (3-ClPA, 21.578 g; 118.2 mmol), phthalic anhydride (0.2664 g; 1.8 mmol), DDS (14.898 g; 60 mmol), sodium phenylphosphinate (50 mg; 0.3 mmol) and o-DCB (85 ml). The reactor was sealed and the temperature was gradually raised. At about 180 C. the reaction mixture was observed to become homogeneous. As water of imidization was removed via the partial condenser the reaction gradually became heterogeneous and eventually the mixture became so thick that mixing of the solid adhering to the quartz window ceased to occur. As the temperature of the reaction mixture was raised further to about 190 C. the heterogeneous reaction mixture began to thin and the viscosity of the heterogeneous mixture was visibly diminished by the time the reaction mixture reached 200 C. Between about 210 C. and about 215 C. most of the solid material present in the reaction mixture dissolved. Thereafter the temperature was raised to 230 C. After about 7 hours imidization was deemed to be complete. Heating was discontinued and as the temperature decreased to about 215 C. the product bisimide began to precipitate from solution. When the temperature had decreased to about 200 C. the product mixture became so thick that efficient stirring could no longer be effected. This Comparative Example illustrates the limitations of “up-front” addition of all of the reactants. Thus, when each of the reactants is added to the reaction vessel at ambient temperature and thereafter the temperature of the reaction mixture is raised. While the reaction mixture is initially homogenous at about 180 C., as water of imidization is removed, the reaction mixture exhibits thick phase behavior which persists until the temperature reaches a higher threshold temperature, in this Comparative Example about 215 C., at which the reaction mixture becomes homogeneous again. The thick phase behavior is undesirable in that it prevents the efficient mixing of the reaction mixture while the thick phase persists and makes accurate measurement of reaction stoichiometry difficult.; Example-1; Imidization in Parr Reactor with Melt CLPA Addition-Procedure:; A 450 ml Parr pressure-rated reactor equipped as in Comparative Example-1 was charged with DDS (14.898 g; 60 mmol), sodium phenylphosphinate (50 mg, 0.3 mmole) and o-DCB (85 mL). The temperature was gradually raised to about 225-230 C. The resultant homogeneous solution was maintained at about 230 C. for 1 hr after which time molten <strong>[117-21-5]3-chlorophthalic anhydride</strong> (3-ClPA, 21.578 g, 118.2 mmole) and phthalic anhydride (0.2664 g; 1.8 mmol) at about 130 C. was added from a stainless steel bomb mounted on top of the reactor. The addition of 3-ClPA was carried out over a period of about 30 to 40 minutes. Water of imidization was azeotropically removed from the reactor via the partial condenser. Throughout the 3-ClPA/PA addition, and thereafter, the reaction mixture was observed to remain a homogeneous solution while at 230 C. No thick phase behavior was observed during the reaction. As the reactor was cooled, the product bisimide was observed to precipitate over a temperature range from about 215 to about 210 C. The reactor was vented when the temperature had decreased to about 170 C. and a sample of the pasty product mixture was withdrawn and analyzed by HPLC. The quality of the product bisimide (“ClPAMI”) was found to be equivalent to the quality of ClPAMI made using known methods.Larger scale imidization reactions using the method of the present invention were conducted in a 5 gallon reactor. The larger reactor was equipped with sampling features which allowed the withdrawal of samples of the reaction mixture as a homogeneous solution without venting the reactor or causing the product bisimide to precipitate during sampling. Examples 2 and 3 are illustrative.A reactor system 10 utilized in various embodiments of the invention is shown in FIG. 1. The reactor system comprised a 5-gallon stainless steel reactor 12 connected directly to monomer and catalyst feed lines 14 and inert gas purge line 15, and indirectly to feed lines 16 for addition of molten reactants. The reactor 12 was connected via distillation line 17 to a partial reflux condenser 18, a total condenser 20 and a back-pressure regulator 22. Feed lines 14 were employed for the batchwise addition of DDS, SSP catalyst, and optionally a portion of the ClPA, whereas, monomer feed lines 16 served for the semi-batch mode introduction of a molten mixture of ClPA and the chain stopper phthalic anhydride (PA). Solvent could be introduced into the reactor via solvent feed line 23.Reactant DDS, ODCB solvent and SPP catalyst were charged to the reactor 12 in batch mode, i.e. prior to starting the reaction. In some reactions a portion of th... |
|
Sodium phenylphosphinate; In 1,2-dichloro-benzene; at 184.1 - 230.7℃; for 0.05 - 2.41667h;Product distribution / selectivity; |
Example 2; The 5-gallon reactor described above and shown in FIG. 1 was equipped with 2 flights of 4-blade pitched turbines, and was charged according to Table I below. TABLE I Component Mass (g) Moles Source 4,4 Diamino Diphenyl Sulfone 1520.8 6.125 Atul (DDS) Phthalic Anhydride (PA) 27.19 0.184 Aldrich Sodium Phenyl Phosphinate 5.0 0.030 Akzo (SPP) 3-Chloro Phthalic Anhydride 2202.8 12.066 Pressure Chem. (ClPA) 1,2 Dicholoro Benzene (ODCB) 9756 66.37 PPG Grade F The reactor was purged with inert gas at 100 SCCM, and the agitation rate was set to 200 rpm. The partial condenser was heated by means of an oil jacket to a temperature of about 130 C. The reactor was heated by setting the reactor oil jacket temperature to 200 C. After 30-minutes of heating, the reactor oil jacket temperature was increased to 230 C. The reactor internal temperature reached a temperature of about 177 C. Water and ODCB were collected as condensed distillate. The reactor pressure was 26.3 psig. Amounts of distillate collected of the course of the reaction, reactor temperature and pressure are shown-in Table II. TABLE II Reactor P Time (min) Reactor T ( C.) (PSIG)H2O (ml) ODCB (ml) 3 184.1 26.3 36 25 6 190.4 26.3 86 52 8 197.4 26.0 114 76 10 205.9 26.4 132 92 16 219.2 25.0 171 117 22 223.5 26.4 186 128 26 225.6 26.4 191 134 30 226.7 26.3 194 138 35 227.7 26.3 198 140 57 230.7 26.2 207 145 After about 1.4 hrs of distillation, the reaction mixture was sampled via the heated sampling port. HPLC analysis of the homogeneous sample indicated that additional 3-ClPA (6.1 grams) was needed to adjust the stoichiometry to within the specified limits. At 2.5 hours of distillation, the 6.1 grams of 3-ClPA was added as a solution of about 9.8 wt % in ODCB.After a total of 4.5 hours the reaction mixture was again sampled. Sample analysis by HPLC indicated that ratio of anhydride to amine was in the range required, from about 1.00 to about 1.03. Therefore, there was no need for any further stoichiometry correction. Heating was continued for a total 9.5 hours at a reactor oil jacket set temperature of 230 C. The reaction was then cooled and diluted with ODCB to provide a slurry of the product so that approximately 3500 grams of solids was obtained as a slurry in 17.8 Kg of ODCB.; Example 3; Melt 3-CLPA Semi-Batch Reaction Mode; The 5-gallon reactor described above and shown in FIG. 1 was equipped with 2 flights of 4-blade pitched turbines, and was charged according to Table III below. TABLE III Component Mass (g) Moles Source 4,4 Diamino Diphenyl Sulfone 1520.8 6.125 Atul Inc. (DDS) Sodium Phenyl Phosphinate 5.0 0.030 Akzo Inc. (SPP) 1,2-Dicholoro Benzene 9991 68.0 PPG Grade F (ODCB) Heated tank 24 (FIG. 1) was charged with phthalic anhydride (PA) and <strong>[117-21-5]3-chlorophthalic anhydride</strong> (3-ClP) in the amounts shown in Table IV and heated to 140 C. in order to melt the 3-ClPA/PA solids. ODCB (240 grams) was then added to the molten mixture of 3-ClPA and PA which was completely miscible with the ODCB. Thus, the mixture in heated tank 24 comprised 3-ClPA (89.2 wt %), ODCB (9.7 wt %), and PA (1.1 wt %). The small amount of ODCB solvent employed helped to facilitate complete transfer of the anhydride reactants into the reaction mixture. TABLE IV Component Mass (g) Moles Source Phthalic Anhydride 27.20 0.184 Aldrich 3-Chlorophthalic Anhydride 2203.0 12.067 Pressure Chem. 1,2-Dicholorobenzene 240.0 1.63 PPG Grade F The 5-gallon reactor was purged with inert gas at 100 SCCM, and the agitation rate was set to 200 rpm. The partial condenser was heated by means of an oil jacket to a temperature of 130 C. The reactor was heated commenced by setting the reactor oil jacket temperature to 180 C. After 30-minutes, the oil jacket temperature was increased to 225 C. At the 1.0-hour mark, the reactor reached a temperature of 226 C., at which point the melt addition of the 3-ClPA-PA-ODCB melt mixture from heated tank 24 was commenced. The reactor pressure was 25.1 PSIG. The 3-ClPA-PA-ODCB melt mixture was pumped via melt feed pump 26 and melt feed line 27 into reactor 12 at an average rate of about 21.5 grams/minute. Addition of the 3-ClPA at a temperature in excess of 210 C. avoided the thick phase reaction stage observed when the reactants are brought into contact at temperatures below about 190 C.About 15 minutes after the addition of the 3-ClPA-PA-ODCB melt mixture was initiated, water and ODCB distillate were observed in the overheads from the partial reflux condenser 18. Addition of the 3-ClPA-PA-ODCB melt mixture required about 2 hours to complete. During the addition of the 3-ClPA-PA-ODCB melt mixture, the amounts of water and ODCB condensed from the overheads emerging from the partial reflux condenser were recorded as a function of time and are recorded in Table V, along with the reactor temperature and pressure profiles. TABLE V Reactor P Time (min)* Reactor T ( C.) (PSIG)H2O (ml) ODCB (ml) 10 222.4 26.5 23 20 20 223.1 26.4 42 33 25 223.2 26.4 53 39 30 223.3 26.5 63 46... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping