| 55% |
With 10 wt% Pd(OH)2 on carbon; hydrogen; In tetrahydrofuran; at 20℃; under 2585.81 Torr; for 16h; |
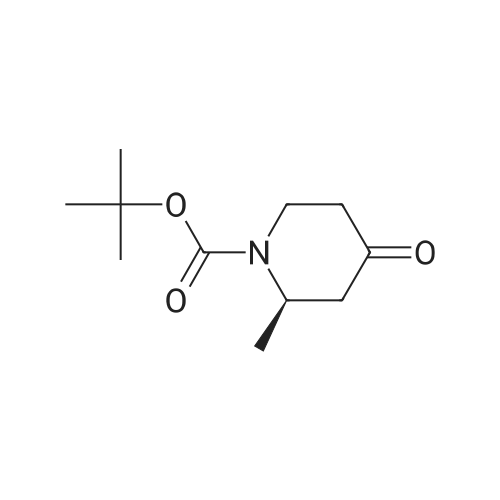
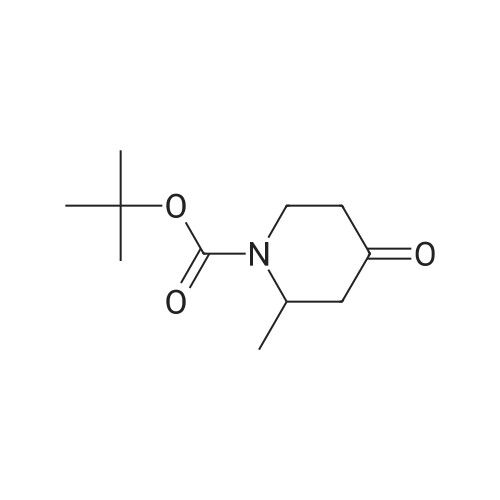
[00239] A mixture of Core-2b_A4b (200 mg, 0.92 mmol), Boc20 (301 mg, 1.38 mmol) and Pd(OH)2/C (50 mg, cat.) in THF (30 ml.) was hydrogenated at 20 C under H2 (50 psi) for 16 h. The reaction was filtered, and the filtrate was concentrated. The residue was purified by column chromatography (PE:EtOAc=4:1) to afford Core-2b_B1 (1 10 mg, yield 55%) as white solid; 1H NMR (400 MHz, CDCI3) d4.64 (brs, 1H), 4.19 - 4.14 (m, 1H), 3.28 - 3.25 (m, 1H), 2.63 - 2.58 (m, 1H), 2.41 - 2.39 (m, 1H), 2.29 - 2.25 (m, 1H), 2.20 - 2.16 (m, 1H), 1.48 (s, 9H), 1.1 1 (d, J = 8Hz, 3H). |
| 15% |
With hydrogen;palladium 10% on activated carbon; In tetrahydrofuran; at 50℃; under 2250.23 Torr; |
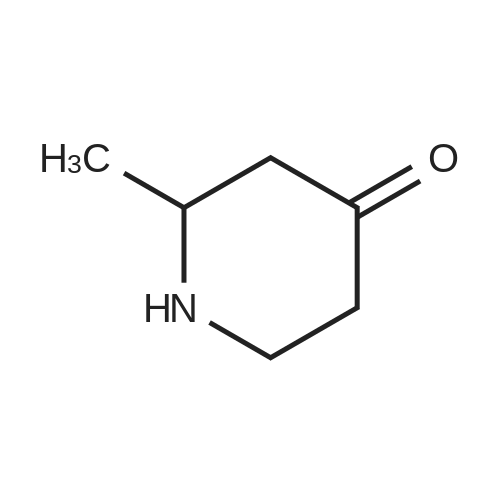
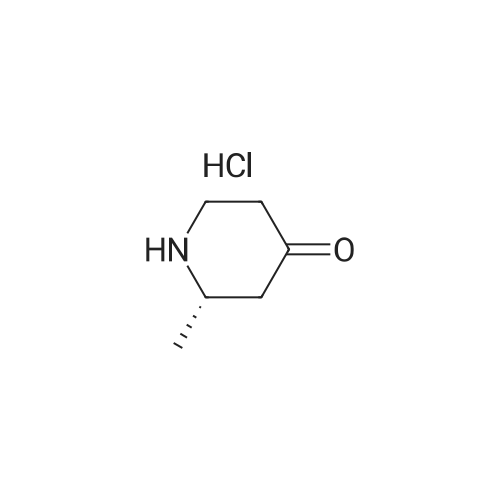
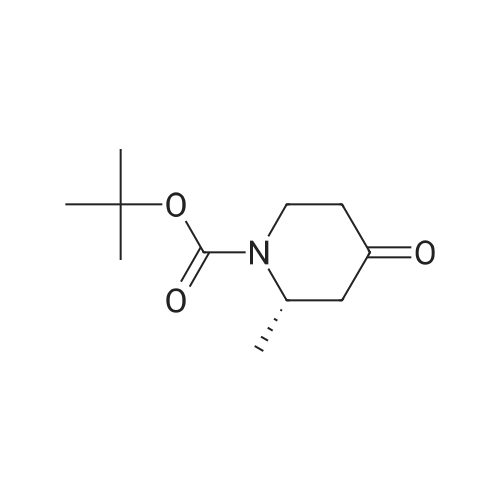
Dissolve 2-Methyl-4-piperidinone hydrochloride (200 g, estimated 1.2855 mole free base content) in water (500 ml). Add the solution to a mixture of methylene dichloride (1 L) and aqueous NAHC03 (180 g in 1.2 L water) at room temperature. Add a solution of di-tert-butyl dicarbonate (280 g, 1.28 mole) in methylene dichloride (500 ML) and stir the reaction mixture overnight at 20C. Separate the water layer and extract twice with methylene dichloride (500ml). Combine the organic layers, wash with water (500 ML), dry over MGS04 (60 g), filter and concentrate under vacuum (276.5 g as red oil). Dissolve the oil in cyclohexane (1 L) and plug-filter through silicagel (300 g). Elute the silicagel with cyclohexane (2L) and 50/50 CYCLOHEXANE/ETHYL acetate (1L). Concentrate the filtrates under vacuum to obtain the title compound as a yellow residue (261 g, 95). Alternatively, N-TERT-BUTYLOXYCARBONYL-2-S-METHYL-4-PIPERIDINONE is prepared in a one-pot synthesis from N- (S)-L-PHENYIETHYI-2-S-METHYI-4-PIPERIDINONE as follows: Dissolve N- (S)-L-PHENYLETHYL-2-S-METHYL-4-PIPERIDINONE (200 g, 0.9208 mole) in THF (200 ml), Add a solution OF DI-TERI-BUTYL DICARBONATE (214. 7g, 0.9838 mole) in THF (200 ML). Place the reaction mixture under a nitrogen flow and add PD/C (10% content, dry catalyst, 10 g). Pressurize the reactor three times with N2, followed by three times with H2. Heat the reaction mixture to 50C and hydrogenate overnight with STIRRING (3 bar H2, ~43. 5 PSI,-300KPA, 300 rpm). Determine the end of the reaction by TLC analysis (silica plate, CYCLOHEXANE/ETHYLACETATE 50/50, complete disappearance of the starting product). Cool the reaction mixture to room temperature and purge the reator by pressurizing three times with N2. Filter off the catalyst using a celite pad and wash with THF (200 ML). Remove the solvent by evaporation (40C, vacuum) to obtain the crude product as a yellow oil (183.5 gram, 93%). Dissolve the crude product in n-hexane (200 ML) and stir overnight at 20C. Filter off the solid, wash with n-hexane (50 ML), and dry under vacuum at 20C to obtain the title compound (78 g, 39%). Concentrate the mother liquors under vacuum to 140 g residual. Cool the solution and stir overnight at 20C. Cool the resulting suspension to 5C and stir for 15 minutes. Filter off the solid, wash with n-hexane (20M1) and dry under vacuum at 20C to obtain additional title compound (30.6 g, 15%). |
|
With hydrogen; palladium(II) hydroxide; In tetrahydrofuran; at 20℃;Inert atmosphere; |
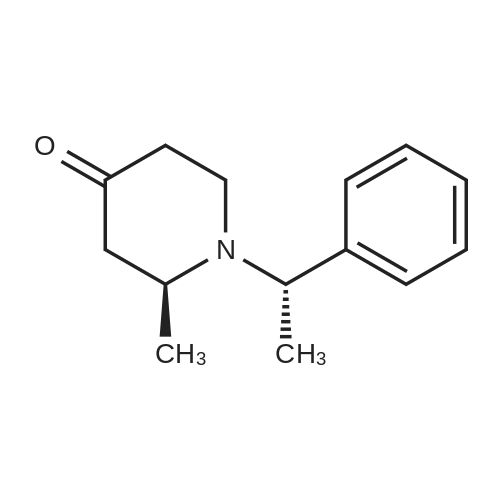
In a 2 neckroundbottomflask (S)-2-methyl-1-((S)-1-phenyl-ethyl)-piperidin-4-one (33) (0.8 g, 3.68 mmol) was dissolved in 18 ml of THF. Boc2O (964 mg, 4.42 mmol) was added under argon. Pd(OH)2 (130 mg, 0.184 mmol) was added and the reaction mixture was hydrogenated overnight at rt. The mixture was filtered over celite, rinsed with THF and evaporated. The crude product was purified by flash chromatography (silica gel, EtOAc/cyclohexane, 10-20%) which furnished the product as white solid. MS (ESI): 214 [M+H]+, 1H-NMR (CDCl3, 400 MHz) delta (ppm): 4.71 (m, 1H), 4.24 (ddd, 1H), 3.32 (ddd, 1H), 2.68 (dd, 1H), 2.48 (ddd, 1H), 2.35 (m, 1H), 2.26 (m, 1H), 1.50 (s, 9H), 1.19 (d, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping