Plasticizer-based and polymer-free ion-selective optodes on cellulose paper
Long Li
;
Carlin Thompson
;
Xuewei Wang
Sensor Actuat. B-Chem.,2024,135925.
DOI:
10.1016/j.snb.2024.135925
More
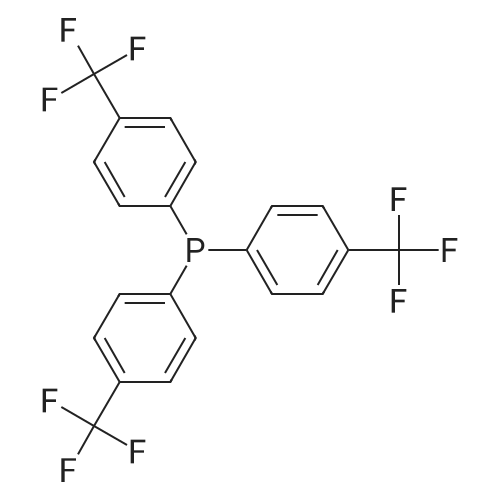
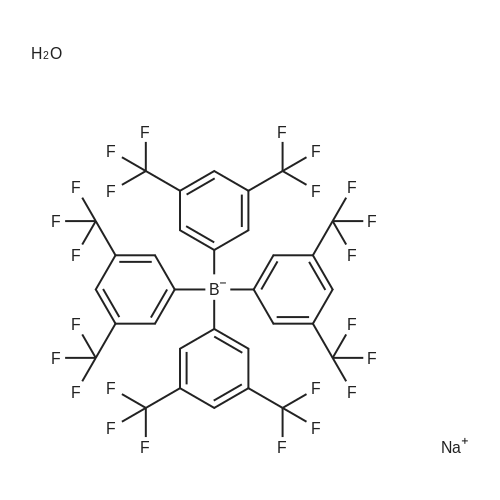
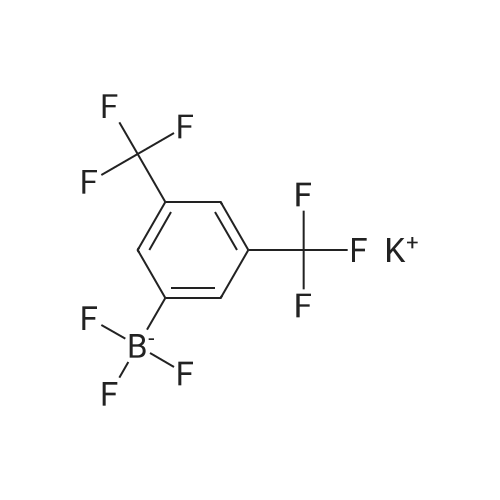
Abstract: Paper-based ion-selective optodes (ISOs) allow for low-cost ion measurements using widely accessible optical detectors such as smartphones, representing an attractive analytical platform for medical testing and environmental monitoring. Previously reported paper-based ISOs rely on plasticized PVC membranes or PVC/plasticizer-free adsorption layers containing hydrophobic sensing chemicals. In this paper, we studied a new format of paper-based ISOs, in which a plasticizer phase containing sensing chemicals is deposited onto cellulose paper without using a hydrophobic polymer like PVC. Compared to ISOs without plasticizers and polymers, the plasticizer-based ISOs show much higher pH sensitivity when using chromoionophore I and tetrakis[3,5-bis(trifluoromethyl)phenyl]borate as the sensing chemicals. When the sensing layer further contains a solid ionophore with high molecular rigidity, the plasticizer-based ISOs for cations also exhibit enhanced sensitivity than the plasticizer-free ISOs. However, when the ionophore is a liquid at room temperature or has a long alkyl chain, the plasticizer-based and plasticizer-free ISOs have comparable responses because the ionophore behaves as a pseudo-plasticizer. Compared to ISOs with both plasticizers and polymers, the elimination of hydrophobic polymers like PVC makes inkjet printing more efficient and preserves the capillary action of the porous paper. By using the newly launched Samba cartridge with smaller nozzles, the plasticizer-based and polymer-free optodes for sodium, potassium, and calcium ions are prepared by inkjet printing for the first time. In an example application, the inkjet-printed plasticizer-based and polymer-free ISOs are used for colorimetric measurements of urine calcium.
Keywords:
Ionophore ;
Ion-selective optode ;
Cellulose paper ;
Inkjet printing ;
Colorimetric
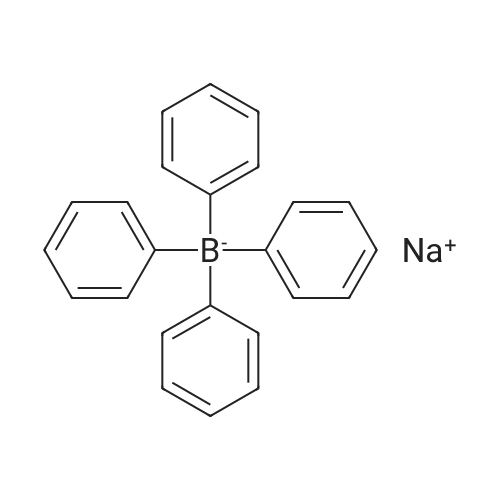
Purchased from AmBeed:
79060-88-1 ;
105560-52-9
Polymerization of Terminal Acetylenes by a Bulky Monophosphine-Palladium Catalyst
Chen, Zhi-Hao
;
Daugulis, Olafs
;
Brookhart, Maurice
Organometallics,2023,42(3):235-239.
DOI:
10.1021/acs.organomet.2c00561
More
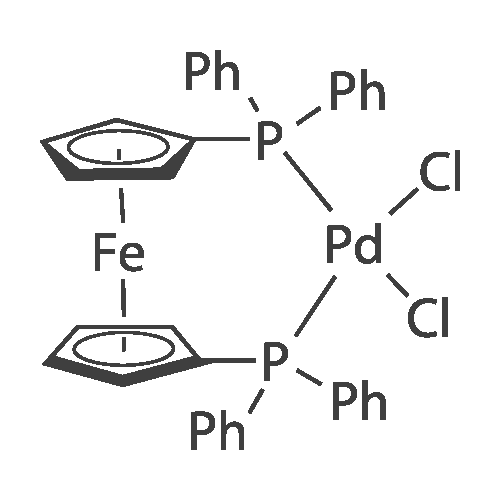
Abstract: A well-defined Pd complex [(tBuXPhos)Pd(Me)(BArf)] bearing a bulky monophosphine ligand was synthesized and fully characterized. X-ray diffraction anal. shows that the 1,3,5-triisopropylaryl group of the dialkylbiaryl phosphine ligand exhibits a weak η6-interaction with the Pd(II) center. This catalyst exhibits excellent functional group tolerance and polymerizes propargyl esters containing benzoyl and acetyl groups, propargyl Me ether, propargyl alc., benzyl acetylene, and Ph acetylene to yield polymers with Mn values at 5-15 kDa with monomodal mol. weight distributions between ~1.4 and 2.1.
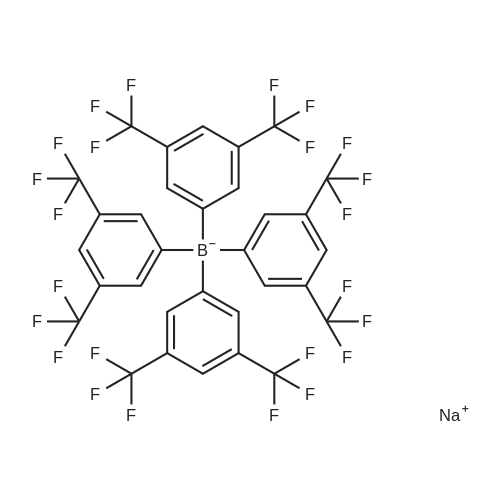
Purchased from AmBeed:
79060-88-1 ;
564483-19-8
Charge‐activated TADDOLs: Recyclable organocatalysts for asymmetric (hetero‐) Diels–Alder reactions
George F. Riegel
;
Keiji Takashige
;
Alex Lovstedt
, et al.
J. Phys. Org. Chem.,2022,e4355.
DOI:
10.1002/poc.4355
More
Abstract: Several charge-containing TADDOL salts were synthesized and used as organocatalysts in asymmetric Diels–Alder and hetero-Diels–Alder reactions. Their catalytic activity was found to exceed that of a noncharged analog while maintaining or improving upon the enantioselectivity. The enhanced activities of the TADDOL salts enabled them to act as presumed hydrogen bond donor catalysts in the Diels–Alder and hetero-Diels–Alder reactions of 1,3-cyclohexadiene with methyl vinyl ketone at 40°C and 2-phenoxy-1,3-butadiene with ethyl glyoxylate at room temperature, respectively. Given the ionic nature of these charge-activated catalysts, it also proved possible to recycle and reuse the TADDOL without chromatography or the need for a recrystallization.
Purchased from AmBeed:
79060-88-1

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping