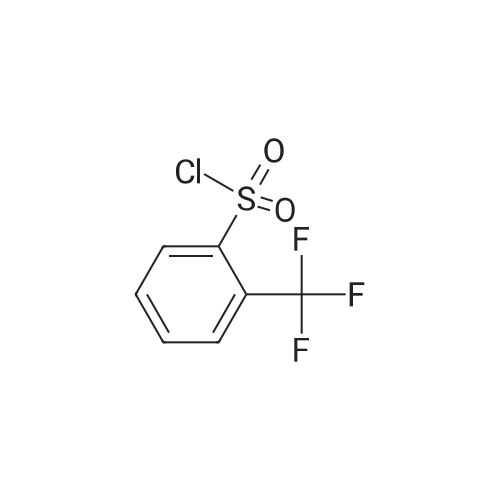
|
With triethylamine; In dichloromethane; at 20 - 37℃; for 48.0h; |
EXAMPLE 1; N-Isopropyl-N-piperidin-4-yl-3 -trifluoromethylbenzenesulfonamide (6); [0480] NaB(OAc)3H (14 g, 66 mmol, Aldrich) was added to a mixture of compound 1 (10 g, 50 mmol, Aldrich), compound 2 (3 g, 52.5 mmol, Aldrich), molecular sieves (4A beads, 2Og, Aldrich) in DCE (200 mL) at 0 0C. The resulting mixture was stirred at room temperature for 24 hours. The reaction mixture was quenched with MeOH (2mL), filtered over celite, washed with water, 2N NaOH and concentrated under vacuum to afford crude compound 3 as a colorless oil. Compound 4 (12 g, 49 mmol, Aldrich) was added to a mixture of the above crude compound 3, TEA (10 mL) and DCM (10 mL) at room temperature. The resulting mixture was heated and stirred at 37 0C for 2 days. The reaction mixture was then cooled to room temperature, washed with water (10 mL), brine, concentrated and purified by column (silica gel, EtOAc/hexanes 3/7) to obtain compound 5 as a sticky oil (10 g, yield 45% in two steps), which was dissolved in 100 mL of 1,4-dioxane. HCl (10 mL, concentrated aq.) was added to the 1,4-dioxane solution at room temperature. The resulting mixture was stirred at room temperature for 48 hours, and concentrated under vacuum. The residue was washed with ethyl ether, and dried to obtain the title compound 6 as HCl-salt, which was suspended in EtOAc, and neutralized with IN <n="152"/>NaOH aq, concentrated and dried under vacuum to give compound 6 as colorless oil (5 g, yield 65%). |
|
With triethylamine; In dichloromethane; at 37℃; for 48.0h; |
Example 1; N-Isopropyl-N-piperidin-4-yl-3-trifluoromethyl-benzenesulfonamide (6); NaB(OAc)3H (14 g, 66 mmol, Aldrich) was added to a mixture of compound 1 (10 g, 50 mmol, Aldrich), compound 2 (3 g, 52.5 mmol, Aldrich), molecular sieves (4 beads, 20 g, Aldrich) in DCE (200 mL) at 0 C. The resulting mixture was stirred at room temperature for 24 hours. The reaction mixture was quenched with MeOH (2 mL), filtered over celite, washed with water, 2N NaOH and concentrated under vacuum to afford crude compound 3 as a colorless oil. Compound 4 (12 g, 49 mmol, Aldrich) was added to a mixture of the above crude compound 3, TEA (10 mL) and DCM (10 mL) at room temperature. The resulting mixture was heated and stirred at 37 C. for 2 days. The reaction mixture was then cooled to room temperature, washed with water (10 mL), brine, concentrated and purified by column (silica gel, EtOAc/hexanes 3/7) to obtain compound 5 as a sticky oil (10 g, yield 45% in two steps), which was dissolved in 100 mL of 1,4-dioxane. HCl (10 mL, concentrated aq.) was added to the 1,4-dioxane solution at room temperature. The resulting mixture was stirred at room temperature for 48 hours, and concentrated under vacuum. The residue was washed with ethyl ether, and dried to obtain the title compound 6 as HCl-salt, which was suspended in EtOAc, and neutralized with 1N NaOH aq, concentrated and dried under vacuum to give compound 6 as colorless oil (5 g, yield 65%). |
|
With triethylamine; In dichloromethane; at 20 - 37℃; for 48.0h; |
EXAMPLE 10; N-Isopropyl-N-piperidin-4-yl-3-trifluoromethylbenzenesulfonamide (19); [0258] NaB(OAc)3H (14 g, 66 mmol, Aldrich) was added to a mixture of compound 14 (10 g, 50 mmol, Aldrich), compound 15 (3 g, 52.5 mmol, Aldrich), molecular sieves (4A beads, 2Og, Aldrich) in DCE (200 ml) at 0 0C. The resulting mixture was stirred at room temperature for 24 hours. The reaction mixture was quenched with MeOH (2ml), filtered over celite, washed with water, 2N NaOH and concentrated under vacuum to afford crude compound 16 as a colorless oil. Compound 17 (12 g, 49 mmol, Aldrich) was added to a mixture of the above crude compound 16, TEA (10 ml) and DCM (10 ml) at room temperature. The resulting mixture was heated and stirred at 37 0C for 2 days. The reaction mixture was then cooled to room temperature, washed with water (10 ml), brine, concentrated and purified by column (silica gel, EtOAc/hexanes 3/7) to obtain compound 18 as a sticky oil (10 g, yield 45% in two steps), which was dissolved in 100 ml of 1,4-dioxane. HCl (10 ml, concentrated aq.) was added to the 1,4-dioxane solution at room temperature. The resulting mixture was stirred at room temperature for 48 hours, and concentrated under vacuum. The residue was washed with ethyl ether, and dried to obtain the title compound 19 as HCl-salt, which was suspended in EtOAc, and neutralized with IN <n="87"/>NaOH aq, concentrated and dried under vacuum to give compound 19 as colorless oil (5 g, yield 65%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping