| 96 - 97% |
With potassium fluoride; In 4-methyl-2-pentanone; at 50 - 80℃; for 5.25h; |
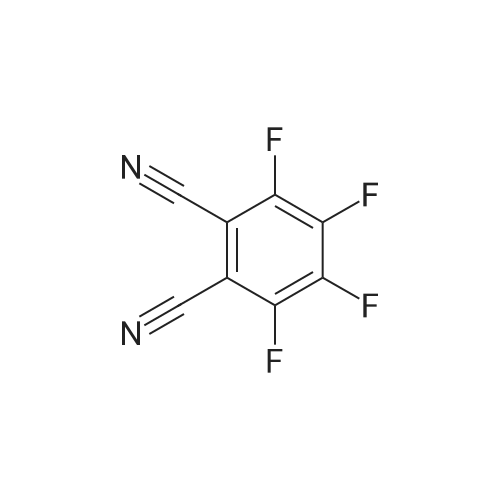
Preparation Example 1 Preparation of 1,4-bis(3,4-dicyano-2,5,6-trifluorophenoxy)tetrafluorobenzene [0080] [C00019] [00019] [0081] Into a 200 milliliter-four necked flask equipped with a stirrer, a cooling reflux tube, a thermometer and a dropping apparatus, 60.52 g (0.30 mol) of 3,4,5,6-tetrafluoro-phthalonitrile, 5.50 g (0.095 mol) of potassium fluoride and 100 g of methyl isobutyl ketone were added, and the mixture was heated to 50° C. A solution formed by dissolving 5.50 g (0.030 mol) of tetrafluorohydroquinone in 9 g of methyl isobutyl ketone was added dropwise from the dropping apparatus over 15 minutes. Then, the mixture was reacted at 50° C. for 2 hours and subsequently at 80° C. for 3 hours. [0082] After the completion of the reaction, a reaction solution was cooled to room temperature and filtered out to separate potassium fluoride, and the like. The obtained filtrate was washed three times with 40 g of 5percent aqueous sodium sulfate solution, and then methyl isobutyl ketone was distilled off. After 50 g of toluene was added to the residue and this mixture was heated to a reflux temperature, it was cooled to room temperature. A precipitated substance was filtered out and a filtered precipitate was washed with 25 g of toluene. By drying this filtered precipitate, 15.77 g (0.029 mol) of the title compound was obtained (yield with respect to tetrafluorohydroquinone: 97percent). A purity of the resulting title compound was measured by liquid-chromatography to give 95percent. [0083] In addition, 45 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> which is a raw material compound remained in the filtrate. By distilling off toluene from the filtrate and further distilling at a distillation temperature of 110° C. under vacuum of 1.3 kPa, 25 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> was recovered. In doing so, since a fluorinated phthalonitrile derivative, which is an intended compound, remained few, a problem of solidification did not occurred. Preparation Example 2 Preparation of 1,4-bis(3,4-dicyano-2,5,6-trifluorophenoxy)tetrafluorobenzene [0084] 15.68 g (0.029 mol) of the title compound was obtained in the same manner as that of Preparation Example 1 (yield with respect to tetrafluorohydroquinone: 96percent). 66 g of toluene and 46 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> were contained in the filtrate after filtering the title compound. [0085] This filtrate was added to a residue obtained by distillation in the above-mentioned Preparation Example 1. By distilling off toluene from the mixture and further distilling at a distillation temperature of 110° C. under vacuum of 1.3 kPa, 45 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> was recovered. 21 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> was contained in a residue by distillation. Comparative Preparation Example 1 Preparation of 1,4-bis(3,4-dicyano-2,5,6-trifluorophenoxy)tetrafluorobenzene [0086] 60.52 g (0.30 mol) of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> and 5.50 g (0.030 mol) of tetrafluorohydroquinone were used as starting materials, and a reaction was performed under the same conditions as that of the above-mentioned Preparation Example 1. [0087] After the completion of the reaction, a reaction solution was cooled to room temperature, and filtered out to separate potassium fluoride, and the like. The obtained filtrate was washed three times with 40 g of 5percent aqueous sodium sulfate solution, and then methyl isobutyl ketone was distilled off. Further, 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong>, which was a starting and was still present excessively, was distilled at a distillation temperature of 110° C. under vacuum of 1.3kPa. By this distillation, 44 g of 3,4,5,6-<strong>[1835-65-0]tetrafluorophthalonitrile</strong> could be recovered, but a residue on distillation was solidified at a time when distillate was not present. A melting point of this residue by distillation was 160° C. or higher. [0088] 20 g of toluene was added to the residue after being distilled off, and after this mixture was heated to a reflux temperature, it was cooled to room temperature. A precipitated substance was filtered and a filtered precipitate was washed with 20 g of toluene. By drying this filtered precipitate, 15.61 g (0.029 mol) of the title compound was obtained (yield with respect to tetrafluorohydroquinone: 96percent). A purity of the resulting title compound was measured by liquid chromatography to give 94percent. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +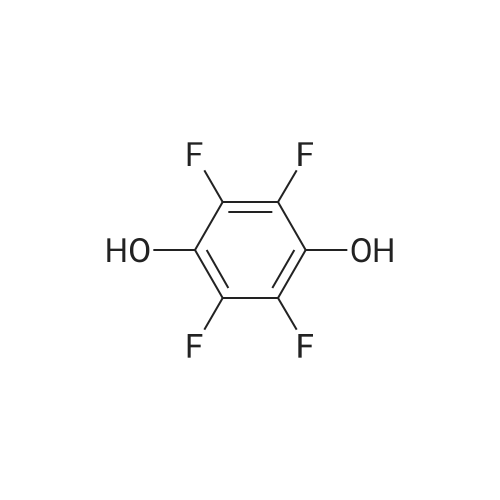

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping