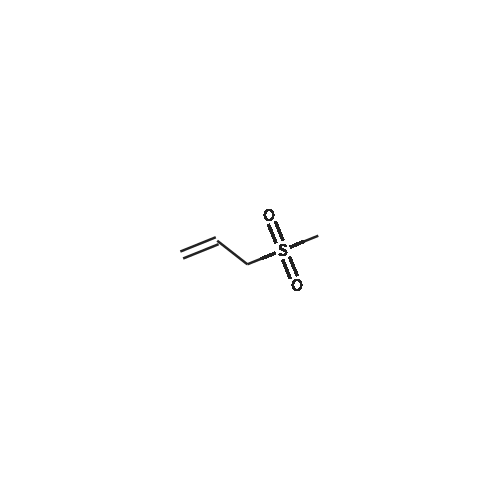
Alternatived Products of [ 77-79-2 ]
Product Details of [ 77-79-2 ]
| CAS No. : | 77-79-2 |
MDL No. : | MFCD00005481 |
| Formula : |
C4H6O2S
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | MBDNRNMVTZADMQ-UHFFFAOYSA-N |
| M.W : |
118.15
|
Pubchem ID : | 6498 |
| Synonyms : |
|
Application In Synthesis of [ 77-79-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 77-79-2 ]
- 1
-
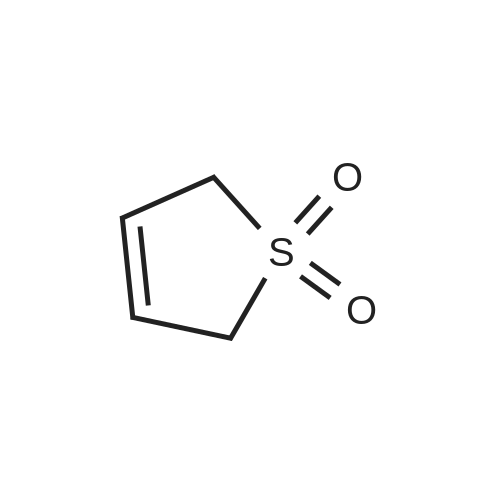 [ 77-79-2 ]
[ 77-79-2 ]

-
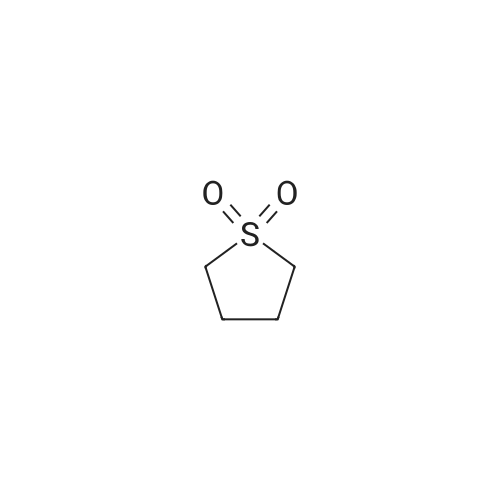 [ 126-33-0 ]
[ 126-33-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogen;Raney nickel; In water; at 35℃; under 7500.75 Torr; for 1.3h;Product distribution / selectivity; |
An amount of 0.186 g (1.0 mmol) of ferrocene was fed into a 500-mL stainless autoclave equipped with a stirrer, a thermometer, a pressure gauge, and a heater. Then, 77 g of sulfur dioxide was charged therein. Next, the autoclave was heated to 100C and 54 g (1.0 mol) of 1, 3-butadiene was injected thereto at a rate of 0.38 g/min using a pump. The mixture was stirred for one hour at 100C. During the stirring, the pressure inside the autoclave was 2.7 to 0.7 MPa. After the pressure inside the autoclave was discharged, 150 g of water was added and the autoclave was cooled to 60C. The autoclave contents were filtered through a filter paper to give a 3-sulfolene aqueous solution. The amount of 3-sulfolene in the obtained aqueous solution was measured using liquid chromatography and the measurement was 103 g (0.87 mol). The yield from 1,3-butadiene was 87%. It is to be noted that polymers were not found on the filter paper used in filtering. All of the obtained 3-sulfolene aqueous solution was fed into a 500-ml conical flask and 70 g of water was added thereto. The solution was warmed to 35C and bubbled with air at a rate of 100 ml/min for one hour. In this manner, sulfur dioxide dissolved in the 3-sulfolene aqueous solution was removed. The concentration of sulfur dioxide in the 3-sulfolene aqueous solution was measured using ion chromatography and the measurement was 31 ppm. Next, 200 g of the obtained 3-sulfolene aqueous solution (3-sulfolene content of 64 g (0.54 mol)) and 1.04 g (0.52 g of pure nickel) of Raney nickel (water content of 50%) were fed into a 500-mL stainless autoclave equipped with a stirrer, a thermometer, a pressure gauge, and a heater. The temperature inside the autoclave was maintained at 35C. Hydrogen was introduced into the autoclave until the pressure gauge read 1.0 MPa. The reaction was initiated while the mixture was stirred at 1000 rpm. Hydrogen was consumed in the hydrogenation reaction and was additionally supplied, when the reading by the pressure gauge lowered to 0.9 MPa, to pressurize to 1.0 MPa. This operation was repeated until the pressure stopped lowering, at which the reaction was determined to be completed. As a result, the reaction time from the start to the completion of the reaction was 78 minutes. After the reaction completed, the reaction rate of hydrogenation was measured using gas chromatography. The measurement clarified that 3-sulfolene was vanished and the reaction was 100% progressed. Table 1 shows the measurements of the amount of generated polymers and the reaction time of hydrogenation. |
Reference:
[1]Recueil des Travaux Chimiques des Pays-Bas,1938,vol. 57,p. 445,455
[2]Recueil des Travaux Chimiques des Pays-Bas,1935,vol. 54,p. 538,540
[3]Journal of the Chemical Society,1951,p. 2556,2561
[4]Journal of the Chemical Society,1958,p. 2888
[5]Industrial and Engineering Chemistry,1949,vol. 41,p. 2635
[6]Patent: EP2368888,2011,A1 .Location in patent: Page/Page column 10-12
[7]Journal of the American Chemical Society,2014,vol. 136,p. 13178 - 13181
- 2
-
 [ 77-79-2 ]
[ 77-79-2 ]

-
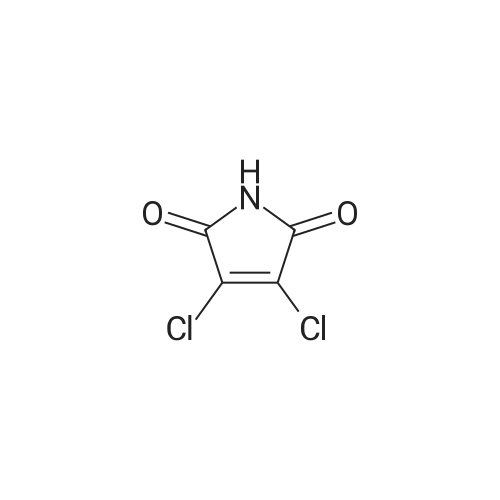 [ 1193-54-0 ]
[ 1193-54-0 ]

-
 [ 2988-66-1 ]
[ 2988-66-1 ]
- 3
-
 [ 77-79-2 ]
[ 77-79-2 ]

-
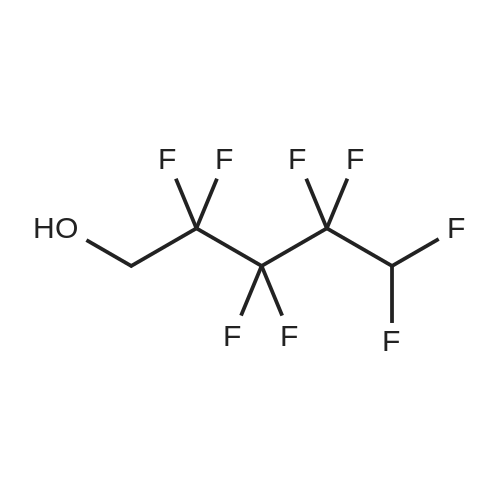 [ 355-80-6 ]
[ 355-80-6 ]

-
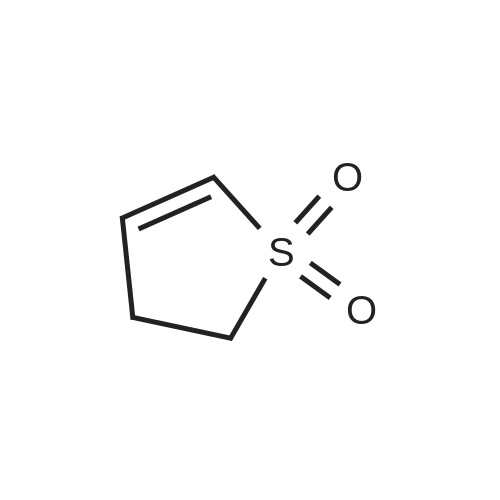 [ 1192-16-1 ]
[ 1192-16-1 ]

-
 [ 59048-70-3 ]
[ 59048-70-3 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping