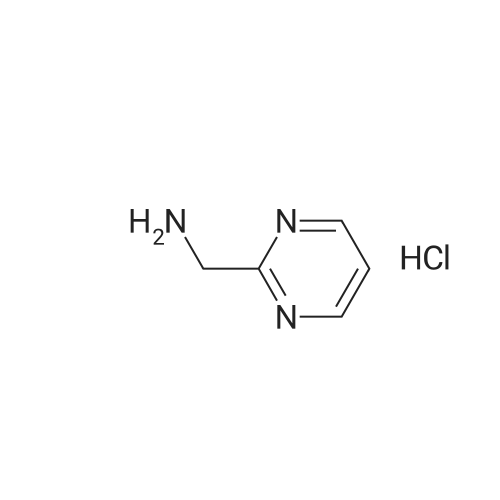
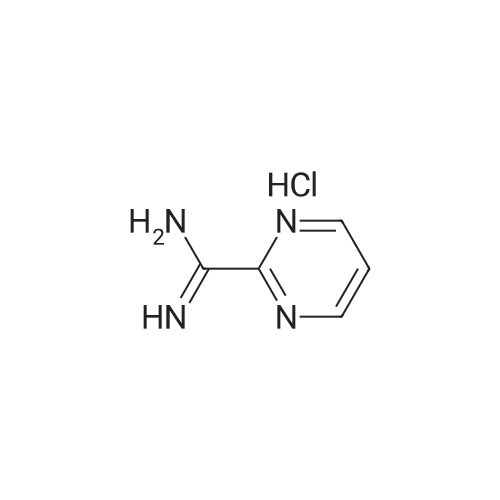
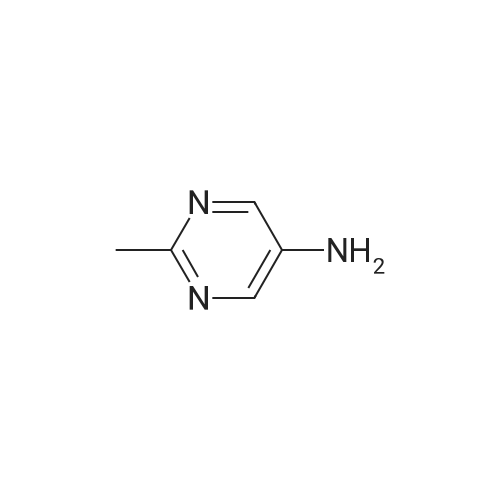
| 99% |
With dicyclohexyl-carbodiimide; 1-hydroxy-1,2,3-benzotriazine-4(3H)-one; In N,N-dimethyl-formamide; at 0℃; |
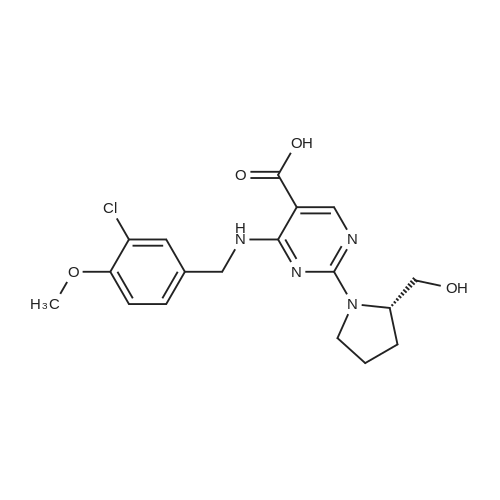
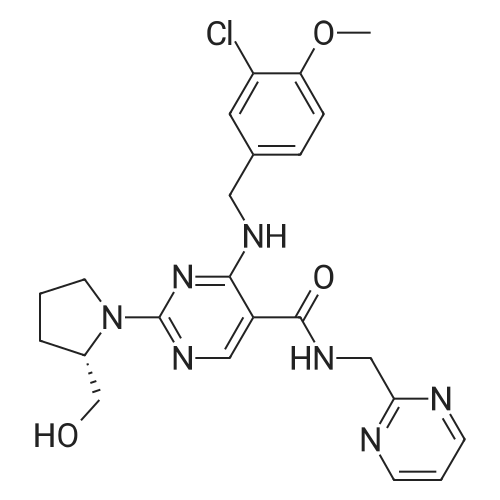
In the reaction flask, anhydrous DMF 3930 mL, compound 5 (393 g, 1.0 mol), 2-aminopyrimidine (163.5 g,1.5 mol), DCC (210 g, 1.02 mol) and 1-hydroxy-1,2,3-benzotriazine-4(3H)-one (166 g, 1.02 mol) at 0 CThe reaction was stirred, monitored until Compound 5 was completely reacted, filtered, and the filtrate was added to water, extracted with chloroform, washed with organic phase and dried.After the filtrate is too short, the silica gel layer is spin-dried to obtain crude avervavir, and the crude avervavir is purified by methanol to obtain pure avenue.The yield was 99.0% and the purity was 99.85%. |
| 91.2% |
With 5,10,15,20-tetrakis[4-(dihydroxyboryl)phenyl]-21H,23H-porphine; In toluene; for 16h;Reflux; Green chemistry; |
4-[(3-Chloro-4-methoxyphenyl)methylamino]-2-[(S)-2-hydroxymethylpyrrol-1-yl]pyrimidine-5-carboxylic acid(39.3g, 100mmol, 1.0eq),2-Aminomethylpyrimidine (12.0 g, 110 mmol, 1.1 eq) and porphyrin borate (7.9 g, 10 mmol, 0.1 eq) were added to 500 mL of toluene.The mixture was heated to reflux to carry out a reaction for 16 hours.After the reaction,Slowly cool the reaction solution to 10-20 C.1000 mL of a 2 wt% aqueous hydrochloric acid solution was added dropwise.The temperature of the control system does not exceed 20 C,Stir for 30min,The boric acid porphyrin catalyst was recovered by filtration.Dispensing the lower aqueous phase,Add 500 mL of dichloromethane to wash the aqueous phase.Slowly add solid NaOH to adjust the pH of the aqueous phase to 7.0, and stir and crystallize for 2 h at room temperature.FiltrationThat is not crude, the filter cake is washed twice with purified water.Add the crude afarafatin to 1000 mL of anhydrous methanol and heat to reflux.After adding 3.0g of activated carbon, stirring and decolorizing for 30min,Hot filtered,The filtrate was stirred and cooled to 30 C, and crystallization was carried out for about 3 hours.filter,After ice-washing with methanol twice, it was dried at 50 C to obtain 44.1 g of a white needle solid (yield 91.2%, purity: 99.63%). |
| 90% |
With benzotriazol-1-ol; dicyclohexyl-carbodiimide; In dimethyl sulfoxide; at 20℃; for 4h; |
To is equipped with a thermometer and constant pressure dropping funnel a 250 ml three-mouth bottle by adding 20g a compound represented by the formula VI, 6.9gHOBT, 6 g2-amine methyl pyrimidine and 100 ml dimethyl sulfoxide; at room temperature to the reaction system under the conditions of adding dropwisely 11g DCC, after dropping, stirring the mixture at room temperature for 4 hours; after the reaction, the reaction system into the dumping 200g ice water, a large amount of solid precipitated, filtered, the filter cake is washed with ethyl acetate to recrystallize, that shall be atorvastatin non -22g, white solid, molar yield is 90%, HPLC purity 99.8%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping