|
With potassium cyanide; sodium hydroxide; In pyridine; methanol; dichloromethane; water; ethylene glycol; N,N-dimethyl-formamide; |
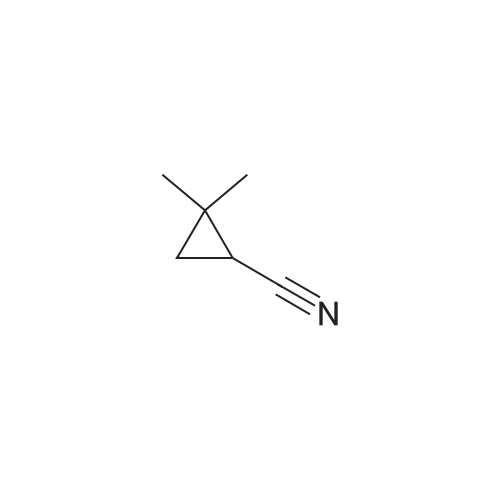
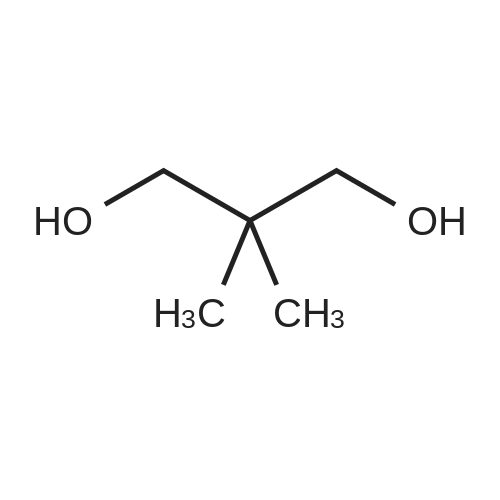
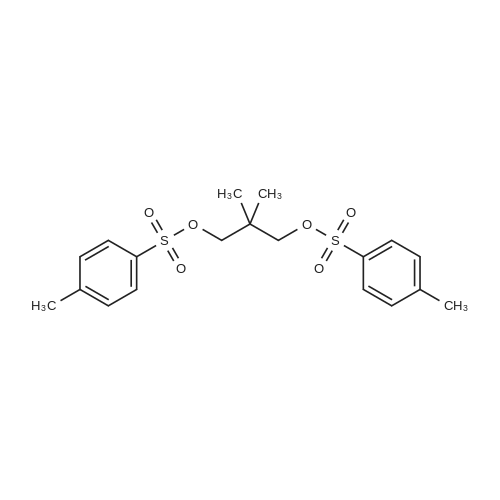
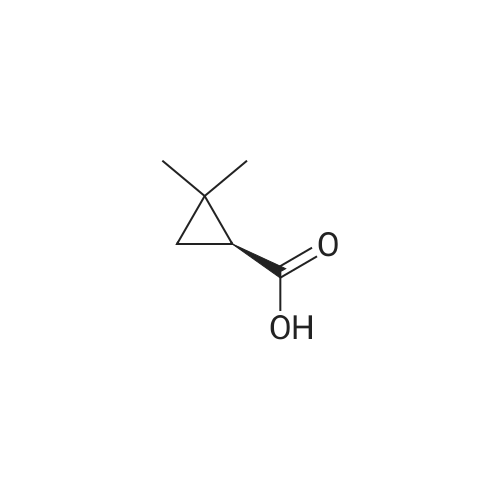
First, 2,2-dimethyl-1,3-propane-ditosylate was synthesised by adding p-toluenesulfonyl chloride (1000 g, 5.25 mol) at 0 C. to a solution of neopentyl glycol (218 g, 2.1 mol) in 500 ml pyridine with stirring. The mixture was stirred for 1.5 hr and then poured into 1500 ml water in a slow stream while stirring vigorously. It was stirred for an additional 1.5 hr and then filtered. The crude solid was recrystallized from acetone (2.0 L), filtered, washed with water (2*0.5 L), hexane (1*0.5 L) and dried. Snow white solid (814 g) m.p. 120-121 C. 2,2-Dimethyl-cyclopropyl nitrile was synthesised by stirring the 2,2-dimethyl-1,3-propane-ditosylate prepared above (412 g, 1.0 mol) with KCN (195.4 g, 3.0 mol) in 2.0 L of ethylene glycol with heating (E. R. Nelson et al., JACS, 1957, p. 3467). At around 80 C., a clear solution was formed. The desired product began to distill out at about 175 C. The distillation was continued until the temperature reached 200 C. The distillate (300 ml) formed two layers. The upper layer was separated and the lower layer was extracted with hexane (3*200 ml). The combined extracts were dried over Na2CO3, concentrated and re-distilled at normal pressure. The yield was 41.7 g (43.8%), b.p. 151-152 C. 2,2-Dimethyl-cyclopropyl carboxylic acid was prepared by mixing 2,2-dimethyl-cyclopropyl nitrile (41.7 g, 0.43 mol) with sodium hydroxide (44 g, 1.05 mol) in water (100 ml) and methanol (50 ml). The mixture was heated to reflux for 48 hr until a clear solution formed. Methanol was distilled off and the aqueous portion was extracted with ether (50 ml) and the aqueous layer was diluted with water (500 ml) and carefully acidified with conc. HCl. The acidified mixture was extracted with ether (5*300 ml), CH2Cl2 (5*300 ml). The extract was evaporated to yield a liquid which was distilled to give 44.9 g (91.6%) of oil, b.p. 55-57 C. at 0.3 mm. 2,2-Dimethyl-cyclopropyl carboxylic acid chloride was prepared by mixing 2,2-dimethyl-cyclopropyl carboxylic acid (20.0 g, 0.18 mol) in CH2Cl2 (100 ml) with 45.7 g (0.36 mol, 31.4 ml) of oxalyl chloride. The mixture was stirred for 1.0 hr and then a small amount of DMF was added to ensure the completion of the reaction. The mixture was then distilled to give 17.8 g (75%) of the desired product, b.p. 84-87 C. Compound III.1, Bis-[4-(2',2'-dimethyl-cyclopropyl carboxy-amido phenol)]-methane, was prepared by combining a solution of 4,4'-methylenedianiline (0.67 g, 3.4 mol) and diisopropyl-ethyl-amine (1.94 g, 2.61 ml, 0.019 mol) in THF (10 ml). The mixture was treated slowly with a solution of 2,2-dimethyl-cyclopropyl carboxylic acid chloride (1.0 g, 7.5 mmol) in THF (10 ml). The reaction mixture was stirred for 1.0 hr and then decomposed with water (250 ml). The precipitated solid was filtered and washed with 10% HCl (10 ml), 10% sodium hydroxide (10 ml), water and ether. The yield was 1.2 g, m.p. 207-210 C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +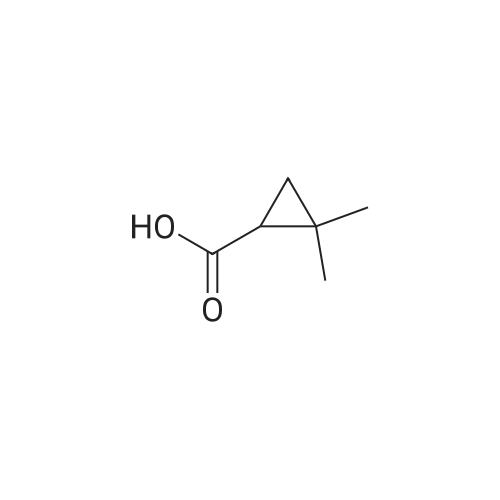

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping