|
|
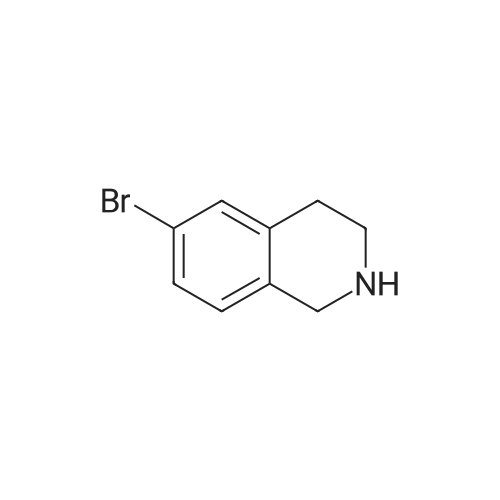
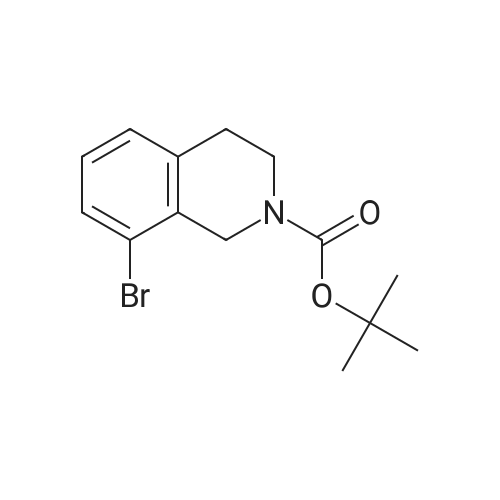
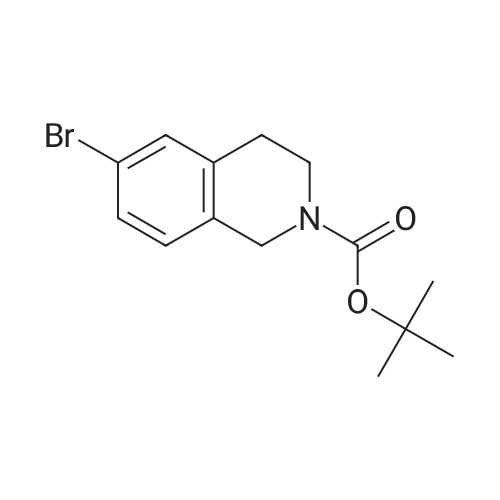
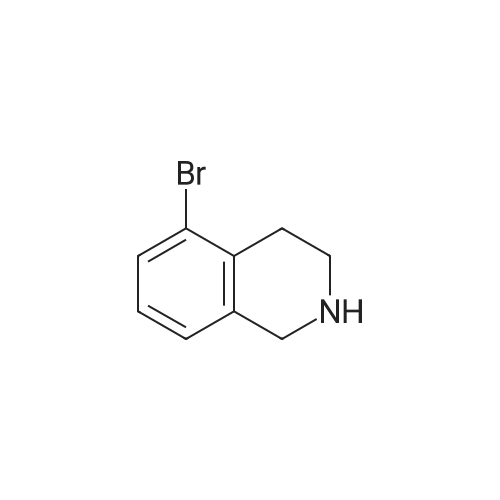
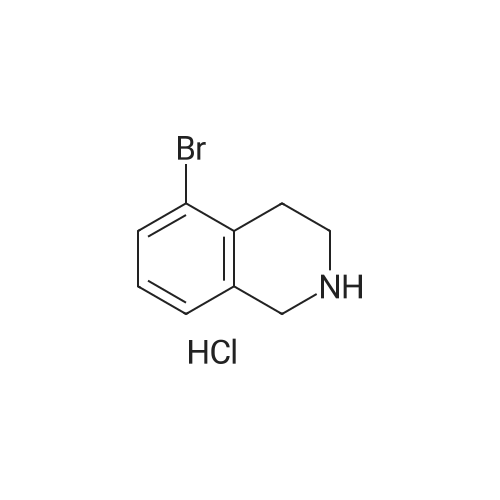
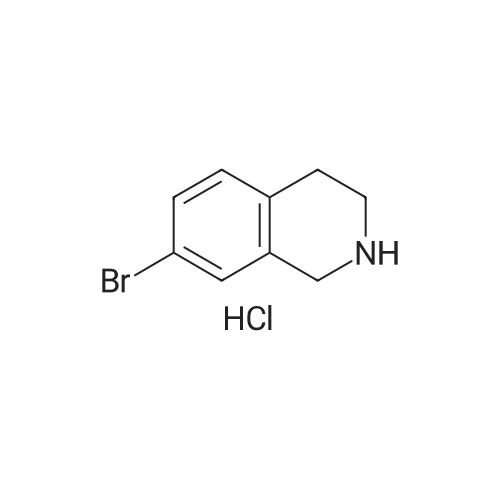
To a mixture of 6-bromo-1,2,3,4-tetrahydroisoquinoline and 8-bromo-1,2,3,4-tetrahydroisoquinoline (22.1 mmol) in THF (100 mL) was added DIPEA (22.1 mmol) and BOC2O (24 mmol). The reaction mixture was allowed to stir at rt over the weekend and then concentrated. Water (5 mL) was added to the residue and the pH was adjusted to 2 by the addition of 1N H3PO4. The mixture was extracted with EtOAc. The organic solutions were combined, dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography to give tert-butyl 6-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate and tert-butyl 8-bromo-3,4-dihydroisoquinoline-2(1H)-carboxylate (6.04 g, 88%) as a yellow oil. |
|
|
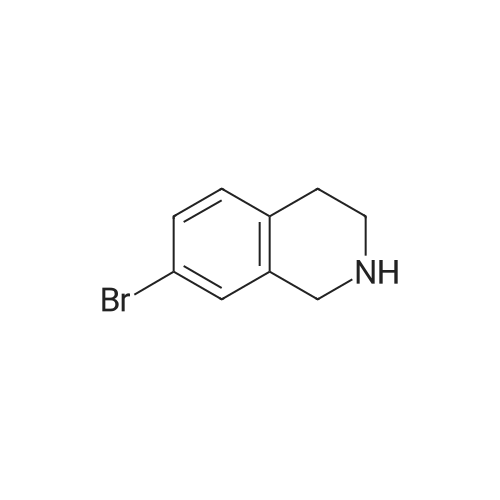
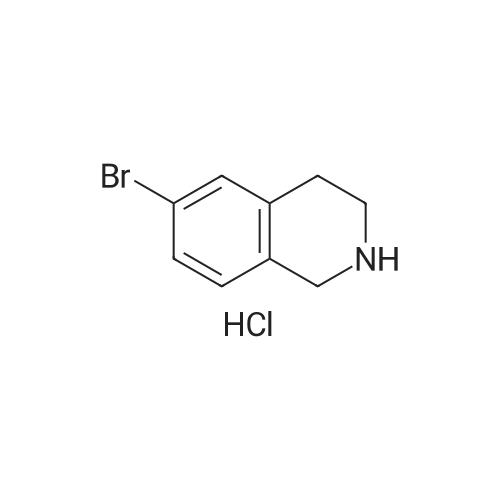
Dry THF (50 mL) and DIPEA (1.3 mL, 7.5 mmol) were added followed by BOC- anhydride (1.8 g, 8.2 mmol). The mixture was stirred overnight at RT. The volatiles were evaporated and the residue was taken up in water. The pH was adjusted to 2 with IM phosphoric acid and the product was extracted twice with EtOAc. The combined organic phases were washed with brine made slightly alkaline with saturated sodium bicarbonate, dried, filtered and concentrated. The crude product was purified by column chromatography with EtOAc-heptanes (1:50 through 1:20) to give 2.24 g (96%) of a 3:1 mixture of the title product and fert-butyl 8-bromo-3,4-dihydroisoquinoline-2(lH)- carboxylate. EPO <DP n="26"/>LC-MS m/z 256/258 (M-56);1H NMR (CDCl3) delta 7.31 (dd, IH), 7.30 (br s, IH), 6.98 (d, IH), 4.52 (s, 2H), 3.63 (t, 2H), 2.81 (t, 2H) and 1.50 (s, 9H) ppm (6-isomer).1H NMR (CDCl3) delta 7.42 (dd, IH), 7.12-7.01 (m's, 2H), 4.55 (s, 2H), 3.64 (t, 2H), 2.84 (t, 2H) and 1.51 (s, 9H) ppm (8-isomerV |
|
|
Dry THF (50 mL) and DIPEA (1.3 mL, 7.5 mmol) were added followed by BOC- anhydride (1.8 g, 8.2 mmol). The mixture was stirred at RT overnight. The volatiles were i5 evaporated and the residue was taken up in water. The pH was adjusted to 2 with IM phosphoric acid and the product was extracted twice with EtOAc. The combined organic phases were washed with brine made slightly alkaline with saturated sodium bicarbonate, dried, filtered and concentrated. The crude product was purified by column chromatography with EtOAc-heptanes (1:50 through 1:20) to give 2.24 g (96%) of a 3:120 mixture of the title product and tert-butyl 8-bromo-3,4-dihydroisoquinoline-2(lH)- carboxylate. LC-MS mlz 256, 258 (M-56);1H NMR (CDCl3) delta 7.31 (dd, IH), 7.30 (br s, IH), 6.98 (d, IH), 4.52 (s, 2H), 3.63 (t, 2H),2.81 (t, 2H) and 1.50 (s, 9H) ppm (6-isomer).25 11HH N NMMRR ( (CCDDCCll33)) delta delta 77..4422 ( (dddd,, I IHH)),, 77..112-7.01 (m, 2H), 4.55 (s, 2H), 3.64 (t, 2H), 2.84 (t, 2H) and 1.51 (s, 9H) ppm (8-isomer). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping