| 76% |
|
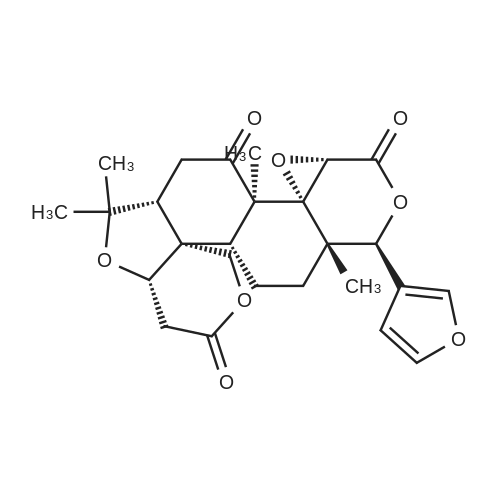
0 C to acetic anhydride (100 mL) Slowly adding chromium trioxide (11 g, 110 mmol), To be stirred until orange, The addition of the raw material Obacunone (45 g, 99.1 mmol) And the temperature was raised to 60 C. The reaction was continued for 3 hours. TLC (PE: EA = 2: 1) was used to monitor the reaction end. After completion of the reaction, the reaction solution was concentrated and the concentrate was separated by ethyl acetate (200 mL) and water (200 mL) The organic phase was dried over magnesium sulfate and concentrated. Intermediate 2 (46.0 g, 98.2 mmol) was dissolved in tetrahydrofuran (200 mL) and methanol (20 mL). Sodium borohydride (6.0 g, 150 mmol) was slowly added at 0 C, TLC (PE: EA = 1: 1) showed the end of the reaction, the reaction was concentrated and the concentrate was extracted with ethyl acetate (200 mL), and the reaction was concentrated at 0 C for 1 hour. ), Washed successively with saturated sodium bicarbonate (100 mL), water (100 mL), and saturated brine (100 mL). The organic phase was concentrated by drying over magnesium sulfate and the remaining crude product was purified by flash silica gel to give intermediate 3 (33.0 g, 70.2 mmol) was dissolved in tetrahydrofuran (100 mL), and the eluent was petroleum ether (PE): ethyl acetate (EA) = 1: 1; And methanol (100 mL) were added lithium hydroxide (2.4 g, 100 mmol). The reaction was stirred at room temperature for 3 hours. TLC (PE: EA = 1: 1) showed that the reaction material disappeared and the reaction product was adjusted to pH = 5 to 6, ethyl acetate (2 x 100 mL). The combined organic phases were dried over magnesium sulfate, filtered and concentrated to give crude intermediate 4 (24.0 g, 71% yield). Intermediate 4 (24.0 g, 49.2 (DE) (10.5 g, 60.0 mmol) was added dropwise to a solution of anhydrous tetrahydrofuran (300 mL) under nitrogen,15.7 g, 60.0 mmol) was added and the reaction was stirred at room temperature under nitrogen for 16 hours. TLC (PE: EA = 1: 1) was monitored and the reaction was complete. After completion of the reaction, the mixture was washed with saturated brine (3 x 50 mL) The organic phase was concentrated by suction over magnesium sulfate and the resulting residue was purified by flash silica gel column to give intermediate 5 (18.0 g, yield 78%). The eluent was petroleum ether (PE): ethyl acetate (EA) 2: 1; Intermediate 5 (18.0 g, 38.3 mmol) was dissolved in anhydrous tetrahydrofuran (200 mL) and a solution of borane tetrahydrofuran (50 mL, 1 M in THF, 50 mmol) was slowly added dropwise. After stirring for 16 hours, H2O2 (50 mL) and saturated sodium bicarbonate solution (50 mL) were added to the reaction solution. The reaction was continued at room temperature for 2 hours. The reaction solution was separated and the aqueous phase was passed through ethyl acetate (3 x 50 mL), the combined organic phases were dried over magnesium sulfate and concentrated by filtration. The resulting residue was purified by flash silica gel column to give product intermediate 6 (11.0 g, yield 58%) eluting with petroleum ether (PE): ethyl acetate (EA) = 4: 1; (DE) (5.9 g, 33.8 mmol), triphenylphosphine (8.9 g, 33 mmol) were added in anhydrous tetrahydrofuran (300 mL) under nitrogen under reduced pressure (11.0 g, 22.5 mmol) 33.8 mmol). The reaction was stirred at room temperature under nitrogen for 16 hours. TLC (PE: EA = 1: 1) was monitored. The reaction was complete and washed with saturated brine (3 x 50 mL). The organic phase was dried over magnesium sulfate The resulting residue was recrystallized from ethanol to give the final product limonin (8.0 g, yield 76%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping