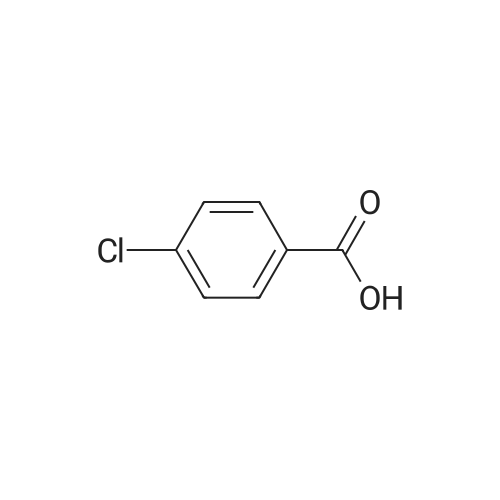
| 87% |
With sulfuric acid; for 48.0h;Heating / reflux; |
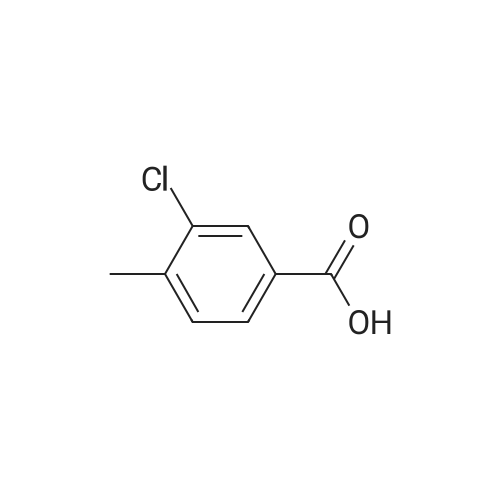
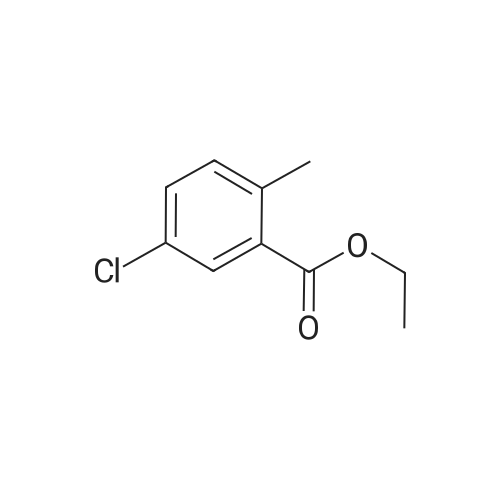
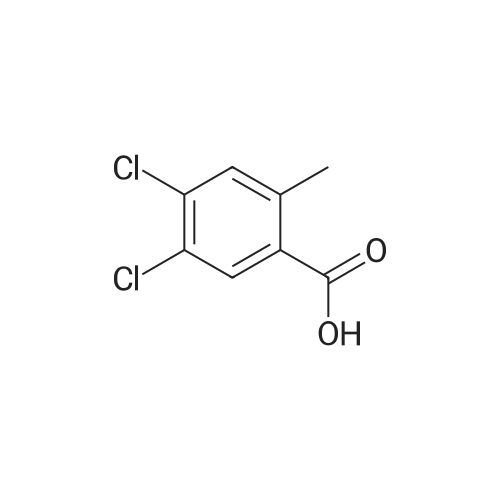
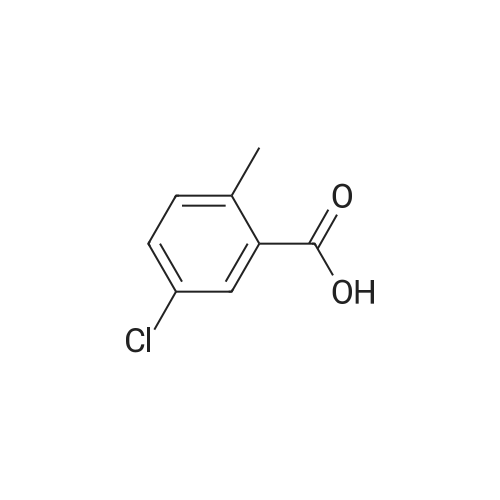
5-chloro-2-methylbenzoic acid (5.04 g, 29.5 mmol) was dissolved in 100 mL ethanol in a 250 mL round bottom flask fitted with a water condenser. 0.5 mL concentrated sulfuric acid was added and the solution heated to reflux. The solution was heated for 48 h and cooled to ambient temperature. The ethanol was removed under reduced pressure. The resultant oil was taken up in 300 mL diethyl ether and washed with saturated aqueous sodium bicarbonate (2 x 300 mL). The organic layer was dried over NA2SO4, filtered and concentrated under reduced pressure to yield 5.12 (87%) of ethyl 5-chloro-2-methylbenzoate as a clear oil. 'H NMR (400 MHz, CDC13) 8 7.88 (d, 1H), 7.35 (dd, 1H), 7.18 (d, 1H), 4.36 (q, 2H), 2.56 (s, 3H), 1.40 (t, 3H). Ethyl 5-chloro-2-methylbenzoate (16.60 g, 83.56 mmol) and diethyl- (3- pyridyl) borane (13.52 g, 91.92 mmol) were dissolved in 100 mL tetrahydrofuran in a 500 mL round bottom flask equipped with a magnetic stirrer. Sodium carbonate (26.57 g, 250.69 mmol) and 50 mL water were added followed by palladium acetate (0.38g, 1.67 mmol) and (2'-dicyclohexylphosphanyl-biphenyl-2-yl)-dimethyl-amine (AmPhos, 0.92g, 2.51 mmol) and 25 mL ethanol. The mixture was heated at reflux for 6 h then cooled to ambient temperature. The mixture was diluted with 600 mL water and extracted with diethyl ether (2 x 300 mL). The organic phases were combined and extracted with 1 N HCI (3 x 200 mL). The acidic extractions were combined and made basic with 5N aqueous sodium hydroxide. This basic layer was extracted with diethyl ether (3 x 500 mL) and the extracts were combined and dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to yield 19.57g (97%) of ethyl 5- (3-PYRIDYL)-2-METHYLBENZOATE as a brown oil. MS (LC-MS) 242.2 (M + H) +. 'H NMR (400 MHz, CDCI3) 8 8.87 (d, 1H), 8.61 (dd, 1H), 8.13 (d, 1H), 7.95 (dd, 1 H), 7.62 (dd, 1 H), 7.44 (dd, 1 H), 7.37 (d, 1 H), 4.40 (t, 2H), 2.65 (s, 3H), 1.42 (q, 3H). A 500 mL hydrogenation vessel was charged with 2. 0g PLATINUM (II) oxide and purged with nitrogen. Ethyl 5- (3-PYRIDYL)-2-METHYLBENZOATE (19.57g, 81.10 mmol) was added as a solution in 200 mL acetic acid. The suspension was hydrogenated at 45 psi for 18 h. The catalyst was filtered through celite and the filter plug was washed with 200 mL acetic acid. The filtrate was concentrated under reduced pressure. The resultant oil was taken up in 500 mL water and made basic with 5N aqueous sodium hydroxide. This basic layer was extracted with ethyl acetate (2 x 500 mL) and the extracts were combined and dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resultant oil was taken up in 200 mL hot ethanol. L- (+)-tartaric acid (12.17g, 81.1 mmol) was added into the ethanol solution and was allowed to stir at ambient temperature for 48 h, forming a white precipitate that was collected by filtration. The white solid was recrystallized from hot 5% H2O/ETHANOL (300 mL) and then from 350 mL hot 20% H20/ETHANOL to yield 11.25g (35%, 95.8% ee) of (S)-ethyl 5- (3-PIPERIDINYL)-2-METHYLBENZOATE-L-TARTARIC acid salt as a white solid. The mother liquors were combined and concentrated under reduced pressure. The resultant oil was taken up in 300 mL water and made basic with 5N aqueous sodium hydroxide. This basic layer was extracted with ethyl acetate (2 x 300 mL) and the extracts were combined and dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resultant oil was taken up in 200 mL hot ethanol. D- (-)-tartaric acid (6.82g, 45.4 mmol) was added into the ethanol solution and was allowed to stir at ambient temperature for 48 h, forming a white precipitate that was collected by filtration. The white solid was recrystallized from hot 5% H20/ETHANOL (300 mL) and then from 350 mL hot 20% H20/ETHANOL to yield 13. 51g (42%, 100% ee) of (R)-ethyl 5- (3-PIPERIDINYL)-2-METHYLBENZOATE-D-TARTARIC acid salt as a white solid. MS (LC-MS) 248.2 (M + H) +. 1H NMR (400 MHZ, CDCI3) 6 7. 73 (d, 1H), 7.24 (dd, 1H), 7.18 (d, 1H), 4.35 (q, 2H), 3.18 (t, 2H), 2.78 (t, 1H), 2.68 (m, 2H), 2.54 (s, 3H), 2.38 (br, 1H), 2.01 (d, 1H), 1.82 (m, 1 H), 1.64 (6,2H), 1.40 (t, 3H). HPLC analysis: Chiralcel AD, 1 mL/min, 10% ethanol/heptane 0.025% diethylamine, rt = 8.36 min, 9.00 min (R)-Ethyl 5- (3-PIPERIDINYL)-2-METHYLBENZOATE-D-TARTARIC acid (2.02 g, 5.08 mmol) was dissolved in 100 mL ethyl acetate and washed with 100 mL saturated aqueous NAHCO3. The organic phase was dried over NA2SO4 and concentrated under reduced pressure. The resultant oil was taken up in 10 mL toluene and imidazole-1-carboxylic acid 4-trifluoromethyl-benzyl ester (1.37 g, 5.08 mmol) was added. The reaction was stirred for 72 h at room temperature under nitrogen. The reaction was diluted with water (200 mL), acidified with 1 N aqueous hydrochloric acid and extracted with diethyl ether (2 x 150 mL). The organic extracts were combined, dried over anhydrous sodium sulfate, filtered and concentr... |
|
With thionyl chloride; at 0 - 70℃; |
Step C: Synthesis of ethyl 5-chloro-2-methylbenzoate as an Intermediate; A mixture of 5-chloro-2-methylbenzoic acid (3.00 g, 17.5 mmol) in ethanol (30 mL) was cooled to 0 C. with an ice bath and thionyl chloride (12.5 mL, 105.5 mmol) was added dropwise, over 30 minutes. After this time, the mixture was warmed to room temperature for 1 h, then transferred to an oil bath, and heated to 70 C. overnight. The mixture was then cooled to room temperature and concentrated under reduced pressure. The solids obtained were then redissolved in diethyl ether (50 mL) and washed with 1 N sodium hydroxide (50 mL), brine (50 mL), dried over magnesium sulfate, and concentrated under reduced pressure to afford ethyl 5-chloro-2-methylbenzoate as a clear oil: 1H NMR (500 MHz, CDC13) delta 7.88 (d, J=2.5 Hz, 1H), 7.34 (dd, J=8.5, 2.5 Hz, 1H), 7.17 (d, J=8.5 Hz, 1H), 4.36 (q, J=7.5 Hz, 2H), 2.56 (s, 3H), 1.39 (t, J=7.5 Hz, 3H); ESI MS m/z 199 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping