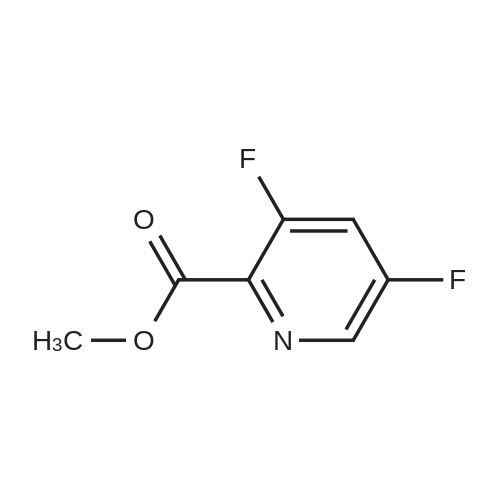
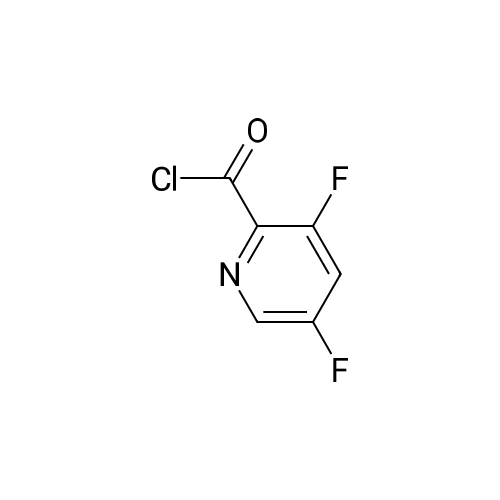
| 100% |
With thionyl chloride;N,N-dimethyl-formamide; In dichloromethane; for 18h;Reflux; |
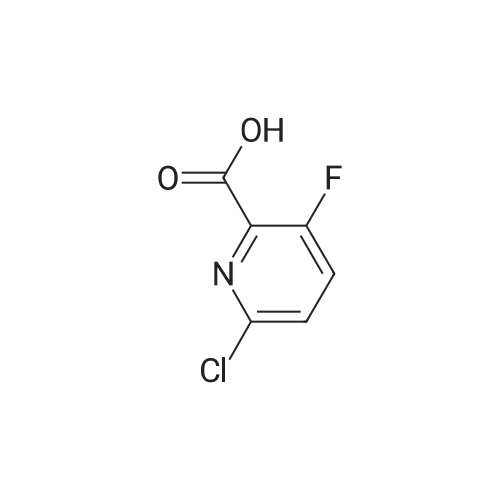
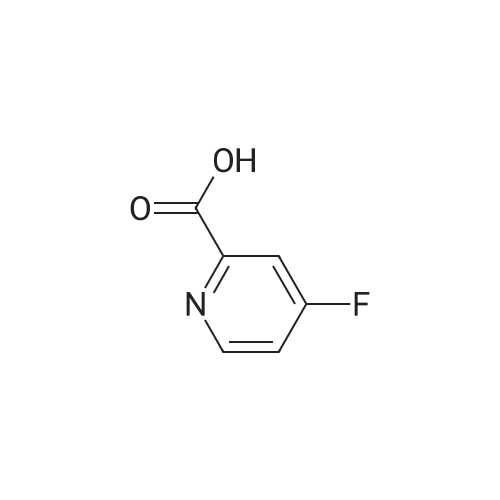
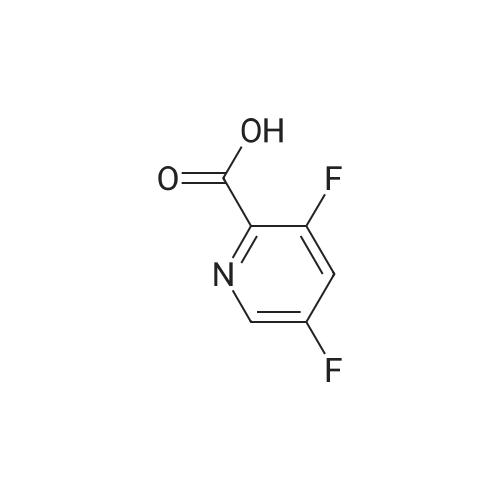
Preparation of 3,5-difluoro-N,N-dimethylpicolinamide (SM-5):(SM-5)<strong>[745784-04-7]3,5-difluoropyridine-2-carboxylic acid</strong> (20.Og, 125.7mmol) was dissolved in dichloromethane (19OmL). Thionyl chloride (46mL, 630mmol) was added, followed by 5 drops of anhydrous N,N-dimethylformamide. The reaction was heated to reflux and stirred for 18 hours. After cooling to room temperature, the reaction was concentrated and azeotroped with dichloromethane to give the desired 3,5-difluoropicolinoyl chloride (22.3g, 100%). 3,5-difluoropicolinoyl chloride (11.2g, 62.9mmol) was suspended in dichloromethane (6OmL) and cooled to O0C. Dimethylamine HCI salt (5.13g, 62.9mnnol) was added. A solution of triethylamine (27.2ml_, 195mnnol) in dichloromethane (2OmL) was then added drop-wise over a period of 3.5 hours. Following the addition, the reaction was allowed to gradually warm to room temperature and stir for 15 hours. The reaction was diluted with saturated sodium bicarbonate and extracted four times with dichloromethane. The combined extracts were dried over magnesium sulfate, filtered, and concentrated. Purification by column chromatography (30 - 100% ethyl acetate in heptane) gave the title compound 3,5-difluoro- N,N-dimethylpicolinamide (SM-5: 10.5g, 89%) as an off-white oil. 1H NMR (400 MHz, CHLOROFORM-d) delta ppm 2.92 (s, 3 H), 3.14 (s, 3H), 7.26 - 7.30 (m, 1 H), 8.34 (s, 1 H). |
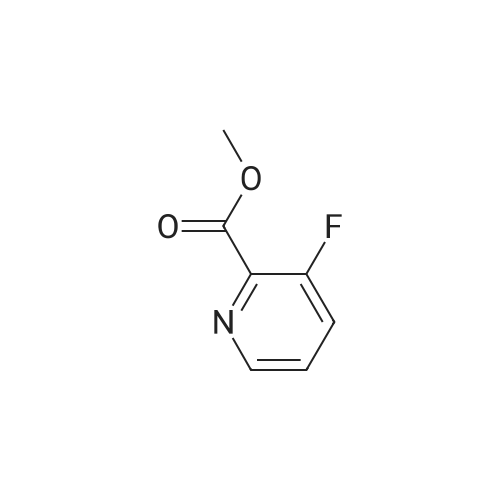
| 100% |
With thionyl chloride;N,N-dimethyl-formamide; In dichloromethane; for 18h;Reflux; |
<strong>[745784-04-7]3,5-difluoropyridine-2-carboxylic acid</strong> (20.0 g, 125.7 mmol) was dissolved in dichloromethane (190 mL). Thionyl chloride (46 mL, 630 mmol) was added, followed by 5 drops of anhydrous Nu,Nu-dimethylformamide. The reaction was heated to reflux and stirred for 18 hours. After cooling to room temperature, the reaction was concentrated and azeotroped withdichloromethane to give the desired 3,5-difluoropicolinoyl chloride (22.3g, 100%). |
| 100% |
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; toluene; at 0 - 20℃; for 2h; |
3,5-Difluoropicolinic acid (51 mg, 320 muiotaetaomicron) was suspended in dichloromethane (5 mL), the suspension was cooled to 0-5C (ice bath) and oxalyl chloride (56.9 mg, 39.3 mu, 448 muiotaetaomicron) as well as dimethylformamide (0.308 M in toluene, 51.9 mu, 16 muiotaetaomicron) were added. The mixture was stirred for 2 h at room temperature. Then, it was concentrated in vacuo (40C, 5 mbar) and dried azeotropically by two cycles of addition of toluene (3 mL) followed by concentration in vacuo to afford 3,5-difluoropicolinoyl chloride as colorless oil (57 mg, quant.). After that, tert- butyl ((3aS,4R,8R)-4-(5-amino-2-fluorophenyl)-4,7,7-trimethyl-8-oxido-3,3a,4,7-tetrahydro-2H- isothiazolo[l,5-a][l,4]thiazin-6-yl)carbamate (Int-16ABp, 80 mg, 188 muiotaetaomicron) was dissolved in dichloromethane (5 mL), the solution cooled to 10C and N,N-diisopropylethylamine (36.5 mg, 49.4 mu, 283 muiotaetaomicron) was added, followed by a solution of 3,5-difluoropicolinoyl chloride (vide supra, 45.4 mg, 256 muiotaetaomicron) in dichloromethane (4 mL). The reaction mixture was stirred for 15 min at 10C. Then, methanol (2 mL) was added, the mixture was stirred for 5 min at room temperature and concentrated in vacuo. The crude was purified by column chromatography (silica gel, 40 g, eluting with 2 M ammonia in methanol / dichloromethane, gradient 1:99 to 4:96) to yield, after drying in vacuo (40C, 5 mbar), the title compound as a white solid (101 mg, 95% yield). HPLC (method LCMS_fglm) tR = 1.23 min. MS (ES+) m/z 566.3 [M+H]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping