| 90% |
|
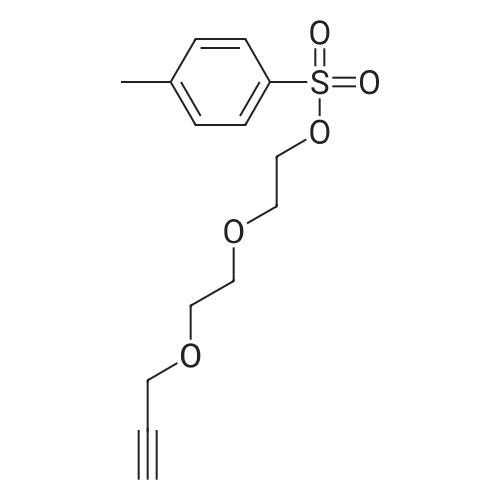
General procedure: In an oven dried REF, monopropargylate (1.0 eq)was dissolved with stirring in TRF at 0 C. Aqueous KOR (4 eq) was added to the flask in small portions immediately. After 10 minutes, tosyl chloride (1.2 eq) solution in TRF was added dropwise and stirred for 12 hours. Upon completion, reaction was quenched with aqueous ammonium chloride and extracted with DCM thrice to get crude product which was purified using silica gel column chromatography using MeORDCM as eluent. The compound 9b was prepared by general procedure 4.1.2, starting from 9a (1 g, 7 mmol), tosyl chloride (1.5 g, 8 mmol), potassium hydroxide (1.3 g, 24 mmol) in THF. The product was obtained as a pale yellow liquid (1.8 g, 6 mmol, 90%) afier purification by silica gel column chromatography using ethyl acetate/hexane as eluent, RrO.55 in 50% ethyl acetate/hexane. ‘H NMR (400 MHz, COd3): OH7.78 (d, J=8 Hz, 2H), 7.32 (d, J=8 Hz, 2H), 4.17-4.13 (m, 4H), 3.70-3.58 (m, 6H), 2.44-2.41 (m, 4H). ‘3C NMR (100 MHz, CDC13): O 144.92, 132.94,129.91, 128.04, 79.57, 77.16, 74.73, 70.59, 69.30, 69.05,68.73, 60.45, 58.48, 21.71, 21.12, 14.31. HRMS (M+Na): 321.07Mol. formula: C,4H,8055Mol. Weight: 298.08Physical appearance: pale yellow liquidYield: 90% |
| 87% |
With sodium hydroxide; In tetrahydrofuran; at 0 - 20℃; |
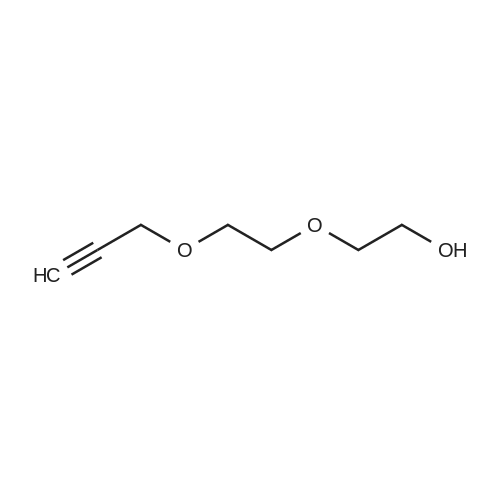
A solution of S3i (1.9 g, 13 mmol) in THF (30 mL) was treated with powdered sodium hydroxide (0.68 g, 17 mmol) at 0 C and toluene sulfonyl chloride (2.7 g, 14 mmol) was added. The reaction mixture was stirred allowing it to warm to room temperature overnight. The solvent was evaporated and the residue taken up in dichloromethane and washed twice with water. After drying over magnesium sulphate the solvent was evaporated and the tosylate purified by chromatography (hexane / ethyl acetate 7:1) to provide a yellow liquid (3.4 g, 87%). The intermediate (3.0 g, 10 mmol) was dissolved in acetone (40 mL) and treated with sodium iodide (3.0 g, 20 mmol). After stirring at room temperature overnight, the solvent was evaporated and the residue taken up in dichloromethane and water. The organic phase was washed with aqueoussodium thiosulfate solution and water and dried over magnesium sulfate. Concentration furnished 9-iodo-4,7-dioxa-nonyne S3 (2.2 g, 88%) as colourless liquid. 1H NMR (400 MHz, CDCI3): O 4.23 (5, 2H), 3.77 (t, 2H), 3.71 (bs, 4H), 3.28 (t, 2H), 2.45 (bs, 1H); 13C NMR (100 MHz, CDCI3): O 79.51, 74.61, 71.98,70.00, 69.05, 58.47, 2.67. |
| 82% |
With dmap; triethylamine; In dichloromethane; at 0 - 25℃; for 16h; |
To a solution of 2-(2-prop-2-ynoxyethoxy)ethanol (2.00 g, 13.8 mmol, Intermediate LC), TEA (4.21 g, 41.6 mmol) and DMAP (170 mg, 1.39 mmol) in DCM (60 mL) was added 4-methylbenzenesulfonyl chloride (5.29 g, 27.7 mmol) at 0 C. The mixture was then stirred at 25 C. for 16 hours. On completion, the mixture was washed with 2.0 M aq.HCl (20 mL) and brine (20 mL), dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by column chromatography on silica gel to give the title compound (3.40 g, 82% yield) as light yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J=8.4 Hz, 2H), 7.35 (m, J=8.0 Hz, 2H), 4.23-4.14 (m, 4H), 3.73-3.68 (m, 2H), 3.67-3.59 (m, 4H), 2.46 (s, 3H), 2.44 (t, J=2.4 Hz, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping