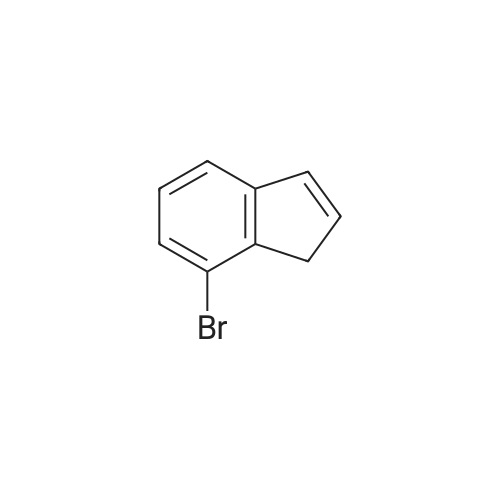
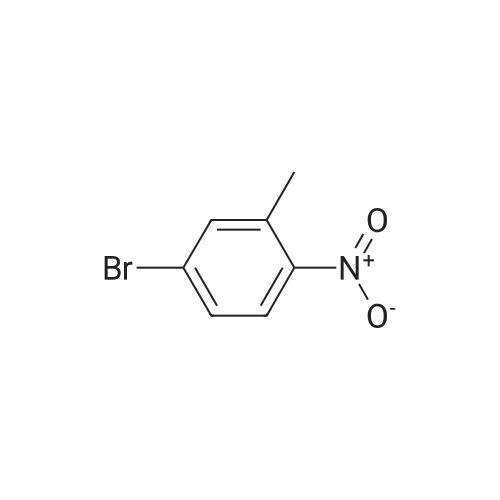
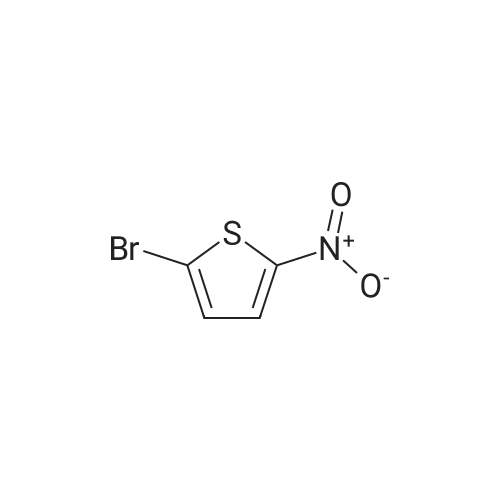
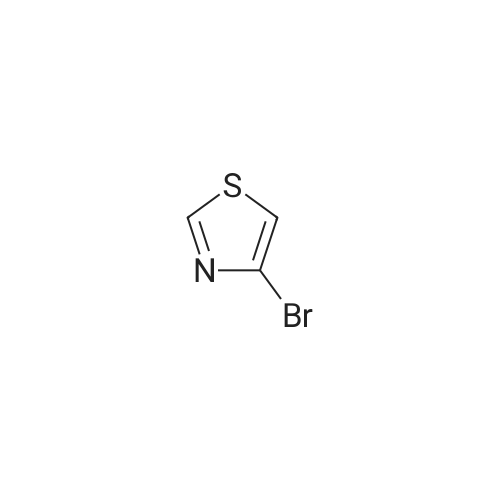
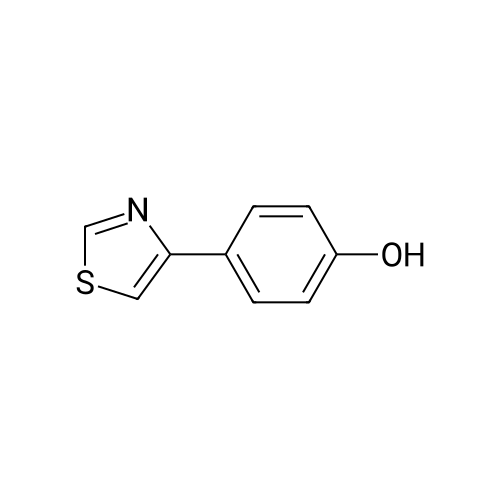
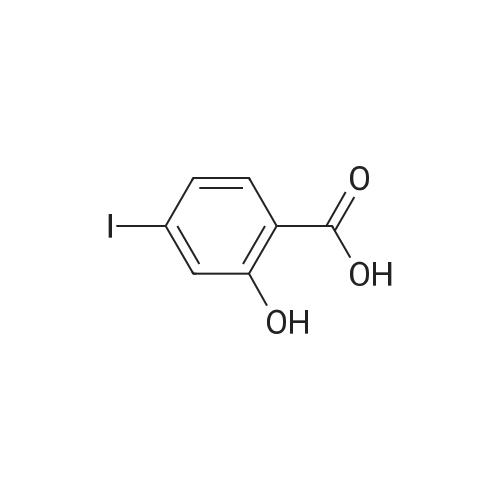
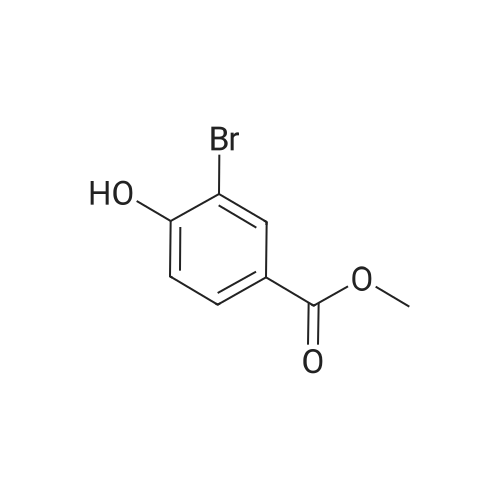
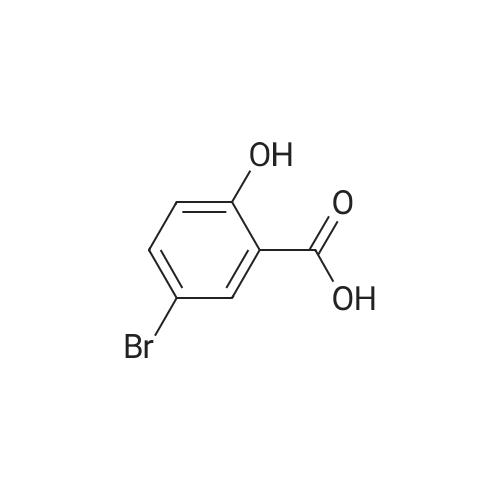
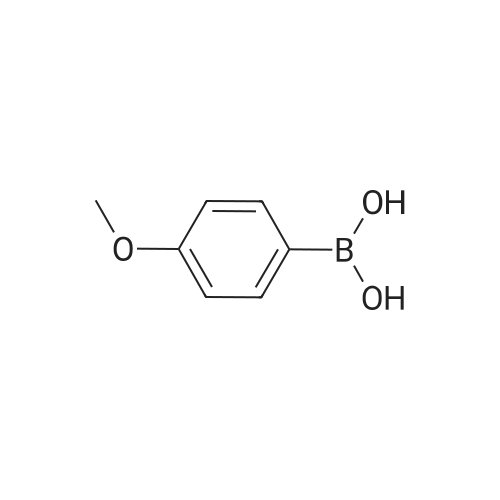
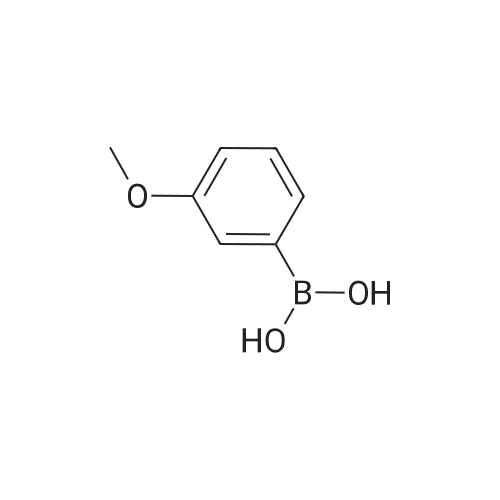
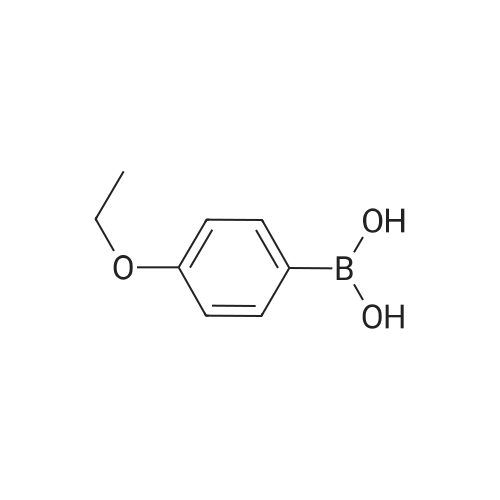
| 100% |
With sodium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In DMF (N,N-dimethyl-formamide); water; at 80℃; for 16h; |
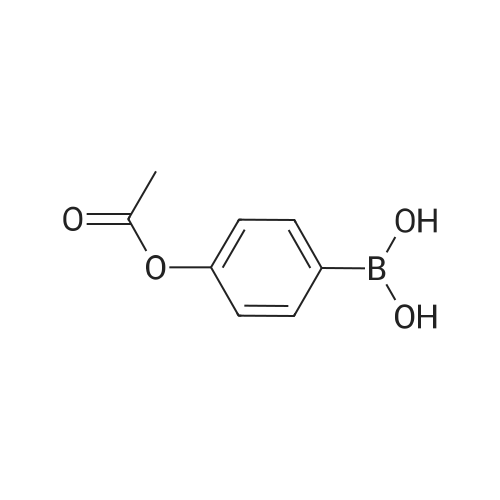
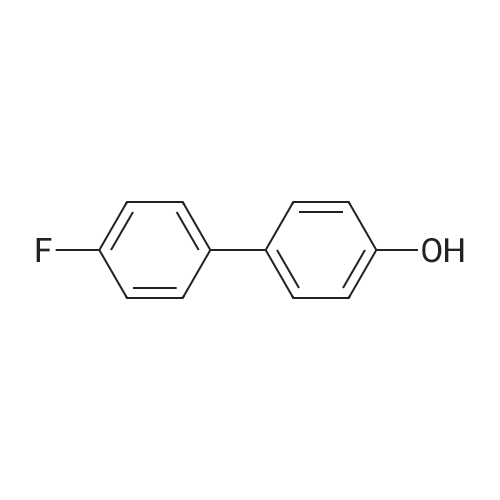
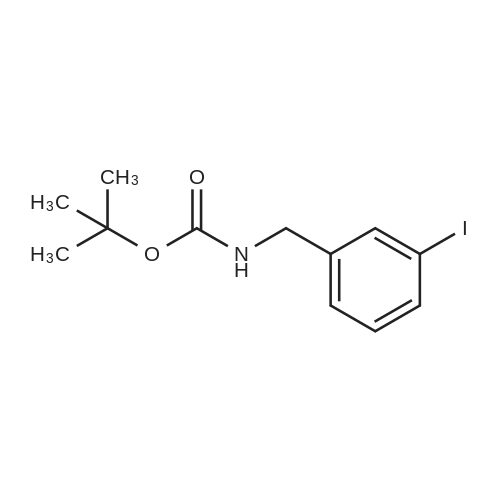
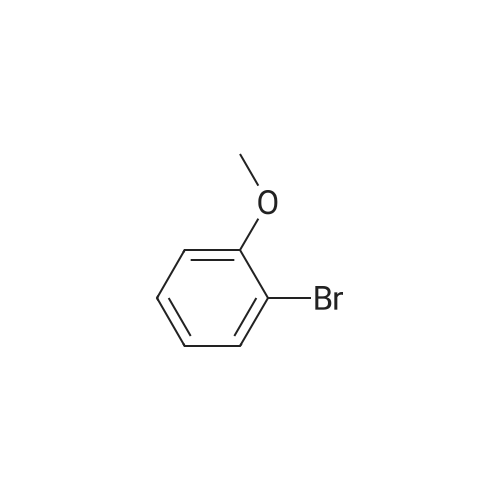
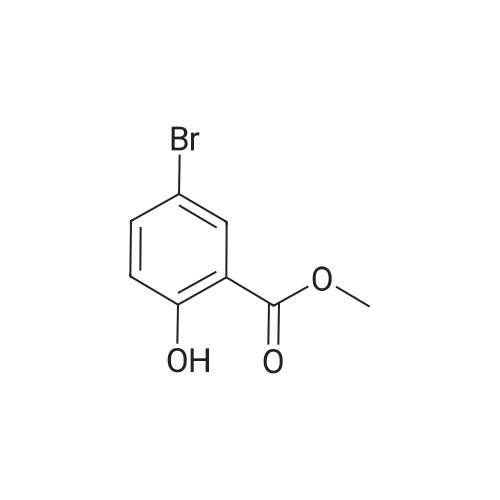
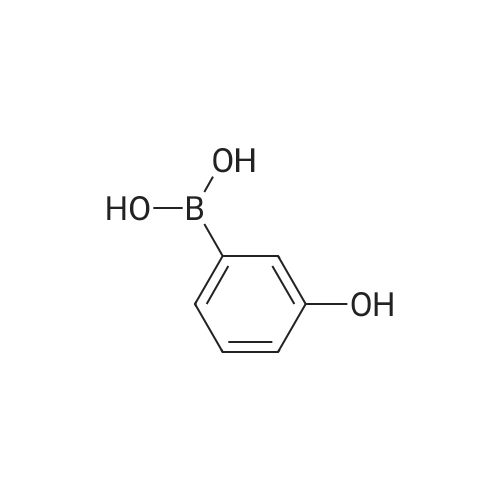
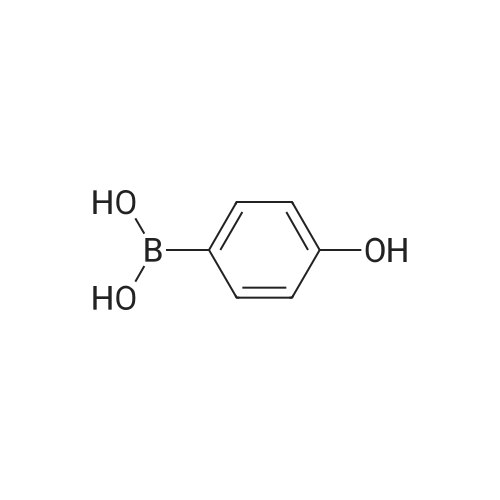
A solution of the product of preparation 49 (0.75 g, 2.25 mmol), 4-hydroxy phenylboronic acid (0.62 g, 4.50 mmol) and 1, 1'- bis (diphenylphosphino) ferrocenyl palladium (II) chloride (0.11 g, 0.14 mrnol) in N, N-dimethylformamide (14 mL) was treated with 2M aqueous sodium carbonate solution (4 mL) and the resulting mixture was heated at 80 C for 16 hours. The solvent was removed in vacuo and the residue was purified by column chromatography on silica gel, eluting with ethyl acetate: pentane, 25: 75, to afford the title compound as a pale pink crystalline solid in quantitative yield, 0. 73 g. 'H NMR (400MHz, CDCI3) 8 : 1.47 (9H, s), 4. 33-4. 41 (2H, m), 4. 87-4. 94 (1H, bs), 6.89 (2H, d), 7.21 (1H, d), 7.37 (1H, dd), 7.43-7. 45 (4H, m); LRMS ESI m/z 298 [M-H]- |
|
With sodium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In DMF (N,N-dimethyl-formamide); water; at 80℃; for 16h; |
A solution of the iodide from preparation 61 (0.75g, 2.25 mmol), 4-hydroxy phenylboronic acid (0.62g, 4.50 mmol), 1,1'-bis(diphenylphosphino)ferrocenyl palladium(II)chloride (0.11g, 0.14 mmol), in N,N-dimethylformamide (14 ml) was treated with 2M aqueous sodium carbonate (4 ml) and the resulting mixture heated at 80 C. under a nitrogen atmosphere for 16 hours. The solvent was removed in vacuo and the residue purified by column chromatography on silica gel eluting with ethyl acetate:pentane (1:3) to give the title compound as a pale pink crystalline solid (0.73g). 1HNMR (400 MHz, CDCl3) delta: 1.47 (s, 9H), 4.33-4.41 (m, 2H), 4.87-4.94 (bs, 1H), 6.89 (d, 2H), 7.21 (d, 1H), 7.37 (dd, 1H), 7.43-7.45 (m, 4H) ppm. MS (electrospray) m/z 298 [M-H]-, 322 [M+Na]+ |
|
With sodium carbonate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In DMF (N,N-dimethyl-formamide); water; at 80℃; for 16h; |
A solution of the iodide from preparation 131 (0.75 g, 2.25 mmol), 4-hydroxy phenylboronic acid (0.62 g, 4.50 mmol), 1,1'-Bis(diphenylphosphino)ferrocenyl palladium(II)chloride (0.11 g, 0.14 mmol), in N,N-dimethylformamide (14 ml) was treated with 2M aq. sodium carbonate (4 ml) and the resulting mixture heated at 80 C. under a nitrogen atmosphere for 16 hours. The solvent was removed under reduced pressure and the residue purified by column chromatography on silica gel eluting with ethyl acetate:pentane (1:3) to give the title compound as a pale pink crystalline solid (0.73 g). 1HNMR (400 MHz, CDCl3) delta: 1.47 (s, 9H), 4.33-4.41 (m), 4.87-4.94 (bs, 1H), 6.89 (d, 2H), 7.21 (d, 1H), 7.37 (dd, 1H), 7.43-7.45 (m, 4H) ppm. MS (electrospray) m/z 298 [M-H]-, 322 [M+Na]+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping