| 98% |
With potassium carbonate; In dimethyl sulfoxide; at 70℃; for 2h; |
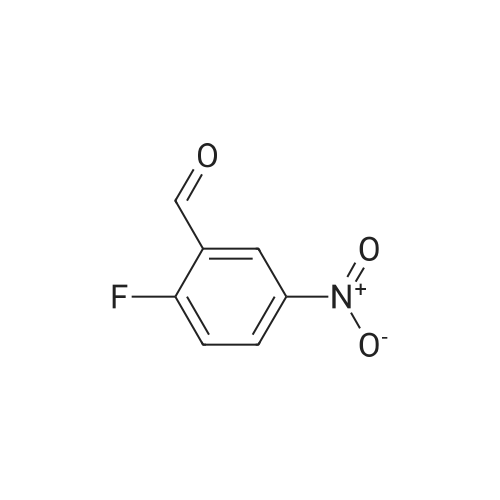
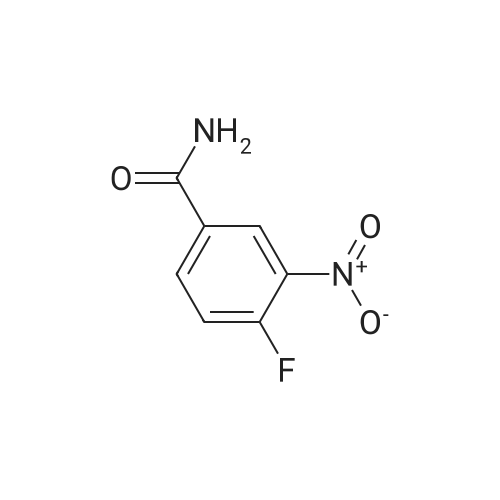
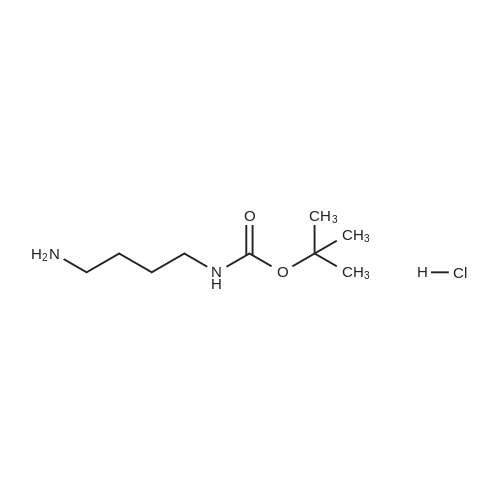
A mixture of tert-buty (4-aminobutyl)carbamate (5.00 g, 26.6 mmol), 4-fluoro-3- nitrobenzamide (4.89 g, 26.6 mmol), and K2CO3 (4.04 g, 29.2 mmol) in DMSO (25mL) was stirred at 70 C for 2 h. The reaction was cooled to rt and slowly diluted with 125 mL of water via addition funnel. The resulting solid was isolated by filtration, dried in a Buchner funnel, and placed in a vacuum oven at 56 C for 3 days to give the title compound (9.2 g, 26.1mmol, 98% yield) as a yellow solid. *H NMR (400 MHz, DMSO-ofe) delta ppm 8.67 (d, J=2.02 Hz, 1 H) 8.40 (t, J=5A3 Hz, 1 H) 8.01 (d, 7=6.82 Hz, 2 H) 7.30 (br. s., 1 H) 7.12 (d, 7=9.09 Hz, 1 H) 6.87 (br. s., 1 H) 3.42 (q, 7=6.57 Hz, 2 H) 2.91 - 3.01 (m, 2 H) 1.60 (d, 7=6.57 Hz, 2 H) 1.43 - 1.54 (m, 2 H) 1.38 (s, 9 H). LCMS (LCMS Method C): Rt.=0.86 min, [M+H]+ = 353. |
| 98% |
With potassium carbonate; In dimethyl sulfoxide; at 70℃; for 2h; |
A mixture of tert-butyl (4-aminobutyl)carbamate (5.00 g, 26.6 mmol), 4-fluoro-3- nitrobenzamide (4.89 g, 26.6 mmcl), and K2C03 (4.04 g, 29.2 mmcl) in DMSO (25mL) was stirred at 70 C for 2 hr. The reaction was cooled to RT and slowly diluted with 125 mL ofwater via addition funnel. The resulting solid was isolated by filtration, dried in a Buchner funnel, and placed in a vacuum oven at 56 C for 3 days to give the title compound (9.2 g, 26.1 mmol, 98% yield) as a yellow solid. 1H NMR (400 MHz, DMSO-o) 3 ppm 8.67 Cd, J=2.02 Hz, 1 H) 8.40 Ct, J=5.43 Hz, 1 H) 8.01 Cd, J=6.82 Hz, 2 H) 7.30 (br. 5., 1 H) 7.12 Cd, J=9.09 Hz, 1 H) 6.87 (br. s., 1 H) 3.42 (q, J=6.57 Hz, 2 H) 2.91 - 3.01 (m, 2 H) 1.60 Cd, J=6.57 Hz, 2H) 1.43 - 1.54 (m, 2 H) 1.38 Cs, 9 H). LCMS (LCMS Method C): Rt =0.86 mi [M+H] = 353. |
| 98% |
With potassium carbonate; In dimethyl sulfoxide; at 70℃; for 2h; |
A mixture of tert-butyl (4-aminobutyl)carbamate (5.00 g, 26.6 mmol), <strong>[349-02-0]4-fluoro-3-nitrobenzamide</strong> (4.89 g, 26.6 mmcl), and K2C03 (4.04 g, 29.2 mmcl) in DMSO (25mL) wasstirred at 70 C for 2 h. The reaction was cooled to rt and slowly diluted with 125 mL of watervia addition funnel. The resulting solid was isolated by filtration, dried in a Buchner funnel,and placed in a vacuum oven at 56 C for 3 days to give the title compound (9.2 g, 26.lmmol,98% yield) as a yellow solid. 1H NMR (400 MHz, DMSO-o) 3 ppm 8.67 Cd, J=2.02 Hz, 1 H)8.40 Ct, J=5.43 Hz, 1 H) 8.01 Cd, 3=6.82 Hz, 2 H) 7.30 (br. s., 1 H) 7.12 Cd, 3=9.09 Hz, 1 H)6.87 (br. s., 1 H) 3.42 (q, J=6.57 Hz, 2 H) 2.91 - 3.01 (m, 2 H) 1.60 Cd, J=6.57 Hz, 2 H) 1.43 -1.54 (m, 2 H) 1.38 Cs, 9 H). LCMS (LCMS Method C): Rt.=0.86 mi [M+H] = 353. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping