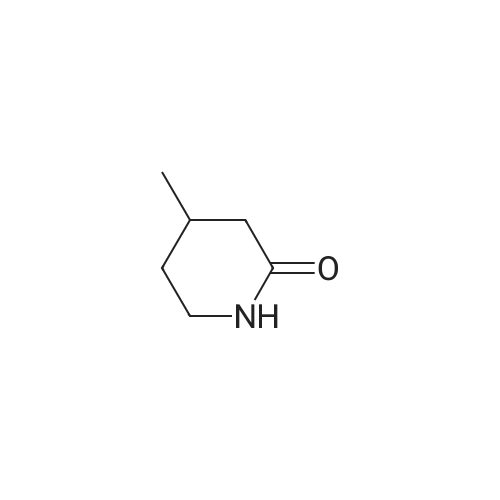
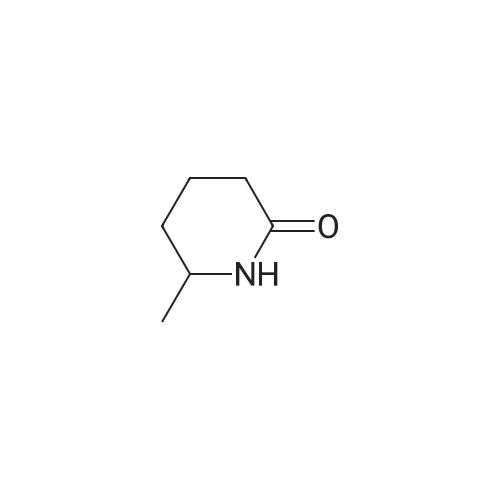
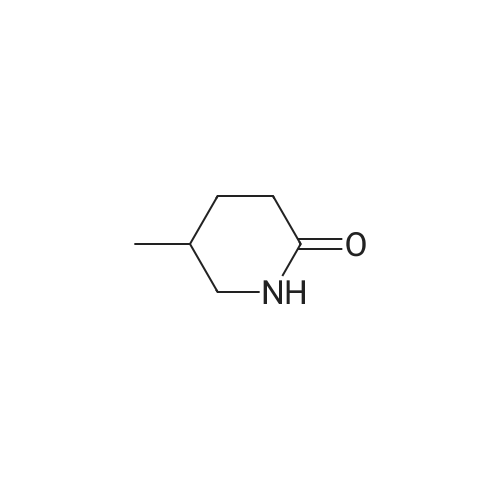
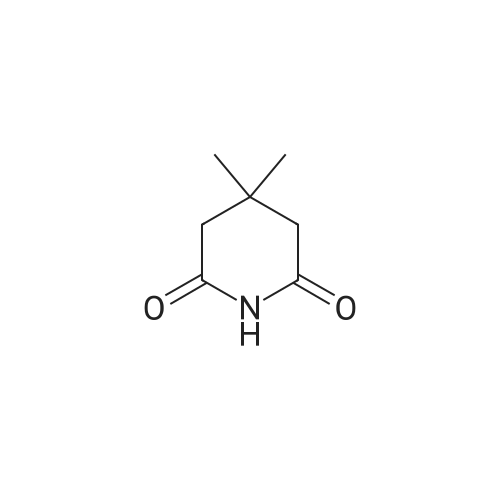
| 99% |
|
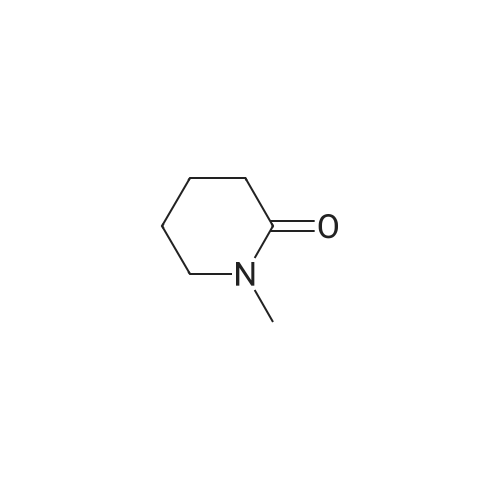
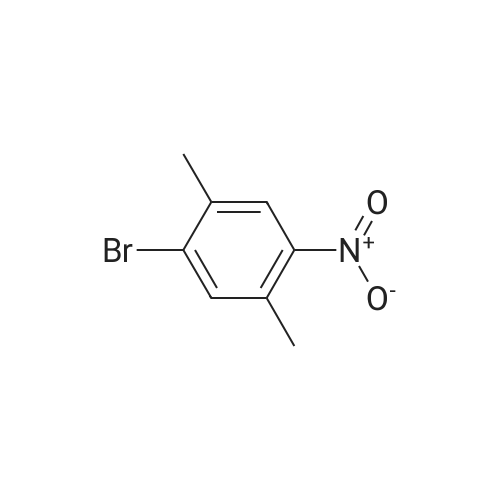
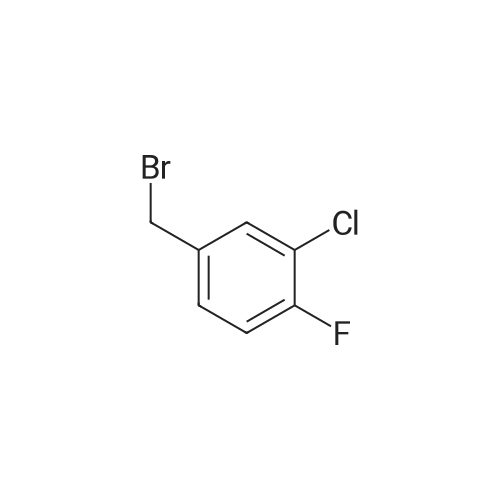
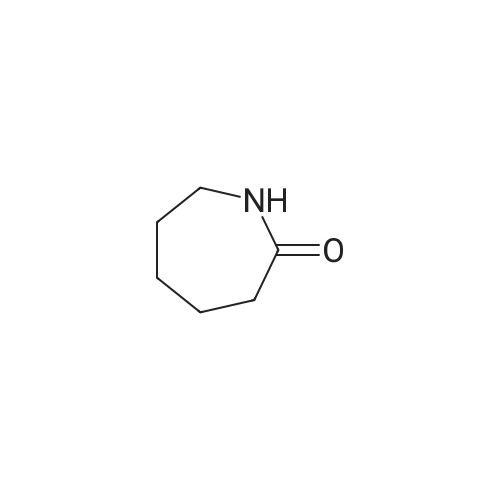
To a 2000 L glass-lined reactor, under the protection of nitrogen, MTBE (633 kg) and valerolactam (34.9 kg, 350 mol) were charged by vacuum. After initiating stirring, a 33% aqueous solution of tetrabutylammonium hydrogen sulfate (8.35 kg) was added. The mixture was cooled to 20-30 C and then a 50% aqueous solution of sodium hydroxide (270 kg) was added to the mixture at a rate of 10-15 L/minute at this temperature. After the addition, the mixture was maintained at the same temperature for 30 minutes followed by the addition of 3- chloro-4-fluoro-benzylbromide (62.9 kg, 280 mol) at a rate of 2-3 kg/minute at 20-30 C. After 5-10 hours, water (283 kg) was added to the reaction mixture at a rate of 30-40 kg/minute at 20- 30 C to quench the reaction. The mixture was stirred for 30 minutes and then the water phase was separated out. The organic phase was washed with 25% aqueous brine solution (226 kg), and the organic phase was dried with anhydrous sodium sulfate (30 kg) under stirring. The dried mixture was filtered by nutsche filter and the filter cake was rinsed with MTBE (50 kg). The combined filtrate was concentrated in vacuo (T< 35 C, P< -0.08 MPa) until the mixture volume remained at about 350-500 L. Petroleum ether was added (138.4 kg) to the mixture and concentrated continuously. After the mixture volume remained at about 350-500 L, another 138 Kg of petroleum ether was added to the mixture and then concentrated in vacuo. The mixture was cooled to 0-5 C, stirred for 2-3 hours, and then filtered. The filter cake was dried by rotary conical dryer below 35 C to provide 67.7 kg of l-(3-chloro-4-fluorobenzyl)piperidin-2-one (99% yield). |
|
|
To a cold (O C) solution of valerolactam (153.30 g, 1.54 mol) in mixture of anhydrous l-methyl-2-pyrrolidinone (3.5 L) and THF (350 mL), sodium hydride (67.7 g, 1.69 mol, 60% dispersion in oil) was added over a period of 5 minutes. The reaction mixture was stirred for 30 minutes, and a solution of 3-chloro-4-fluorobenzylbromide (345.5 g, 1.54 mol) in l-methyl-2-pyrrolidinone (200 mL) was added over 30 minutes at 0 C. The reaction mixture was stirred at 0 0C for 1 hour, and was allowed to warm up and stirred at room temperature overnight. The reaction mixture was quenched with distilled water (5 L), and extracted with dichloromethane (three times; 2 L, 1 L, 1 L). The organic extracts were combined, washed with <n="20"/>water (3X; 4 L each time). The residual oil was dissolved in ethyl acetate (4 L), and extracted with water (3X; 2 L each time). The organic layer was separated, concentrated under vacuum to give the title product that solidified upon standing. lH NMR (400 MHz, CDCI3) delta 7.24 (m, 2H), 7.0 (m, 2H), 7.1 (m, IH), 4.56 (s, 2H), 3.19 (t, J=4.9 Hz, 2H), 2.46 (t, J= 6.4 Hz, 2H), 1.8-1.75 (m, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping