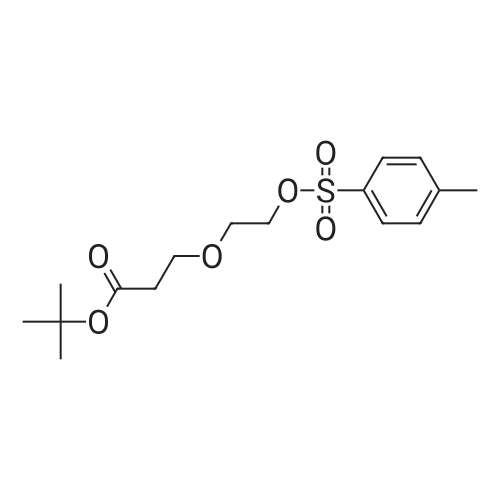
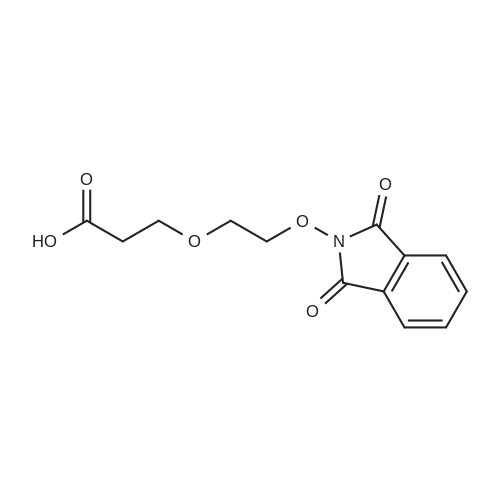
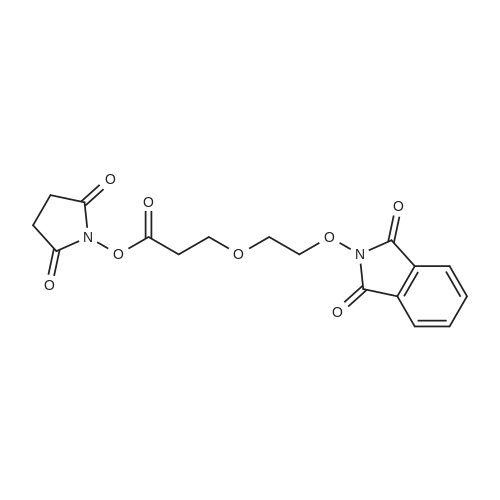
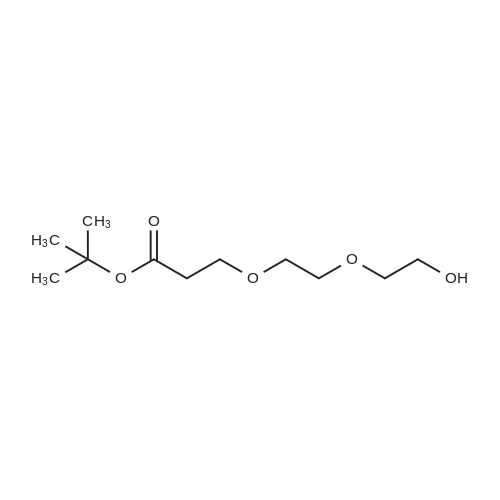
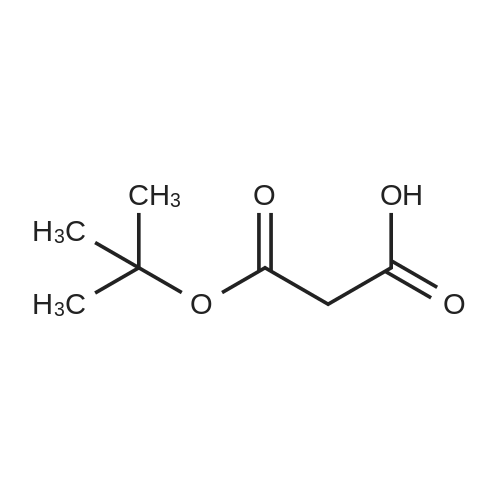
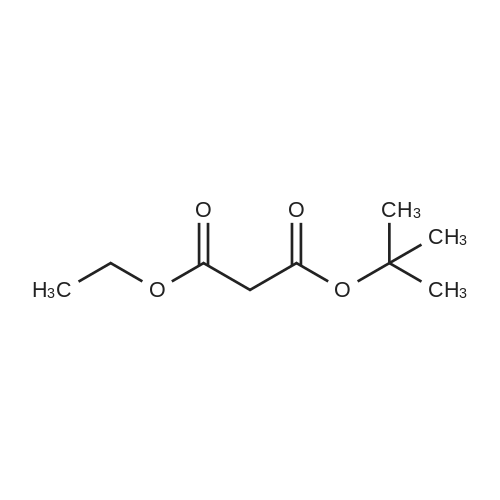
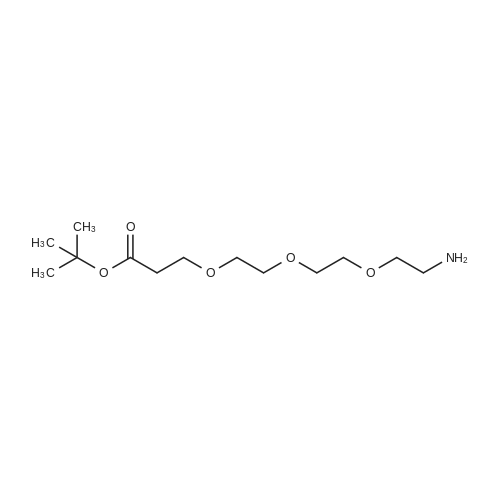
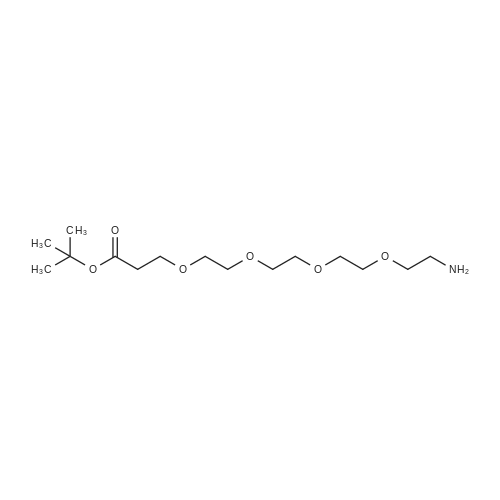
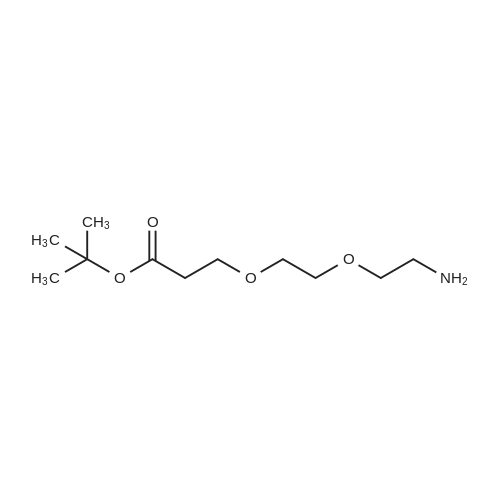
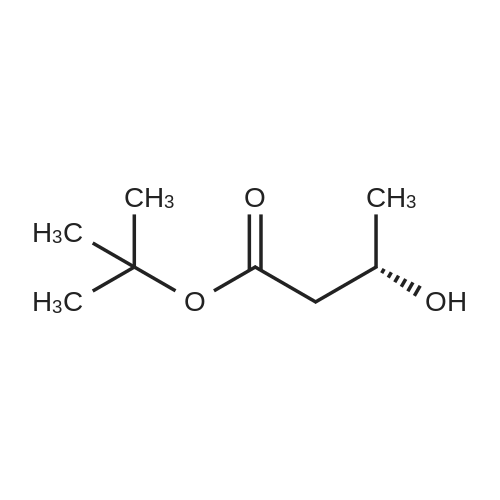
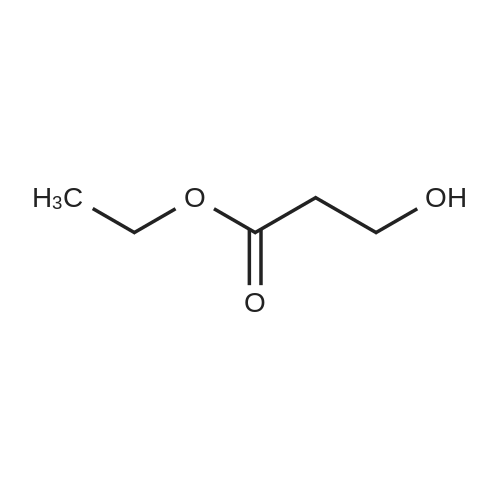
| 22% |
With tetrabutylammomium bromide; potassium hydroxide; at 20℃; for 96h; |
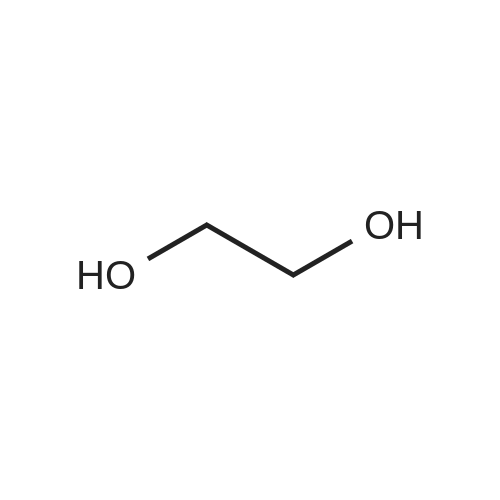
tert-Butyl acrylate (5.72 mL, 39.0 mmol) was added to a mixture of ethylene glycol (2.42 g, 39.0 mmol), tetrabutylammonium bromide (377 mg, 1.17 mmol) and potassium hydroxide (77 mg; purity, 85%; 1.17 mmol), and the mixture was stirred at room temperature for 4 days. The reaction mixture was purified by silica gel column chromatography (hexane:ethyl acetate = 4:1 ? 2: 1, v/v) to give the title compound (1.66 g; yield, 22%) as a colorless oily substance. 1H NMR (CDCl3, 400 MHz): delta 1.47 (9H, s), 2.44 (1H, brs), 2.52 (2H, t, J = 6.1 Hz), 3.59 (2H, t, J = 5.6 Hz), 3.73-3.76 (4H, m). |
| 6.9% |
|
To a solution of ethylene glycol (5.0 mL) in THF (100 mL) was added sodium hydride (0.19 g, 4.7 mmol) at 0 C., and then the reaction mixture was stirred warming to room temperature for 30 minutes. To the reaction mixture at 0 C. was added a solution of tert-butyl acrylate (2 g, 16 mmol) in THF (50 mL) over 30 minutes, and the mixture was stirred at room temperature for 2 days, and concentrated in vacuo. The obtained residue was purified by silica gel column chromatography (pentane:ethyl acetate=3:1-1:1) to give Compound (g-2) (0.34 g, 6.9% yield). (1086) 1H-NMR (CDCl3, 400 MHz) delta: 4.21-4.20 (m, 2H), 3.87-3.82 (m, 1H), 3.75-3.70 (m, 2H), 3.59-3.56 (m, 2H), 2.52-2.49 (m, 2H), 1.46 (s, 9H). |
|
With potassium hydroxide; tetrabutylammomium bromide; at 20℃; |
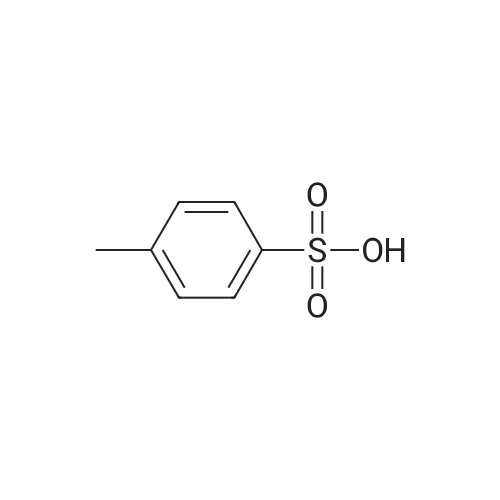
Tert-butyl acrylate (130 g, 1.01 mole) was added dropwise over 3 hours to a mixture of anhydrous ethylene glycol (62 g, 1.0 mole), tetrabutylammonium bromide (9.6 g) and KOH (powder, 2.2 g), and stirred overnight at room temperature under an argon atmosphere. The volatile products were distilled off under reduced pressure (rotoevaporator, 60 C.) and the mixture was dissolved in 250 ml dichloromethane. The solution was washed with 250 ml of distilled water, dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The product (Compound 2) was then subjected to vacuum distillation (kugelrohr, t=95-100 C., 0.05 mm Hg). Yield 36.6 g. NMR (d6-DMSO): 1.40 ppm (s, 9H), 2.42 ppm (t, 2H), 3.39 ppm (m, 2H), 3.46 ppm (m, 2H), 3.59 ppm(s, 2H), 4.55 ppm (t, 1H). |
| 14 g |
|
Ethanediol (7.6 mL, 0.15 mol) was dissolved in 100 mL dry THF, 100 mg metal sodium was added, the reaction mixture was stirred at room temperature till the metal sodium was completely consumed, tert-butyl acrylate (14.5 mL, 0.1 mol) was added, then the mixture was stirred overnight at room temperature. THF was removed under reduced pressure, 100 mL EtOAc was added to the residue, the mixture was washed with water for 3 times (50 mL×3), dried over anhydrous sodium sulfate, concentrated, the crude product was purified by silica gel column chromatography (PE/EtOAc=1:1) to give 14 g product 2-22 as colorless oil, yield 73%. LCMS (ESI) m/z 191.1 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping