| 61.7 - 66.8% |
|
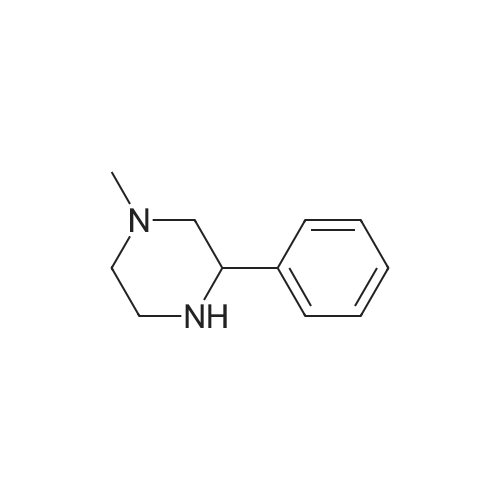
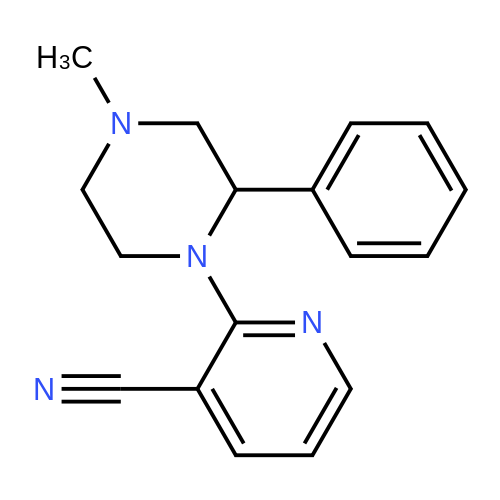
Example 1 2-(4-methyl-2-phenylpiperazin-1-yl)-3-cyanopyridine oxalate After adding 21.1 g (119.7 mmol) of <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong>, 20.0 g (144.4 mmol) of 2-chloro-3-cyanopyridine, and 16.6 g (164.1 mmol) of triethylamine to 42 g of dimethylformamide, the mixture was reacted at 125 to 130 °C for 24 hours under a nitrogen atmosphere. After distilling out triethylamine and dimethylformamide from the reactant solution under a reduced pressure, the residue was added with 32 ml of water and 87 g of ethyl acetate, and then a pH value thereof was adjusted to 8 to 9 with 10 percent aqueous sodium hydroxide solution. After phase-separating the solution, an organic layer was added with 24 g of methanol, and then 15.2 g of oxalic acid. This solution was filtrated to collect crystals, and then the crystals collected were dried to obtain 31.6 g of an objective compound (HPLC content: 86.1 percent, the yield from <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong> was 61.7 percent). IR (KBr) gamma=3039, 2223, 1733, 1636, 1578, 1567, 1436, 758, 701 cm-11H-NMR (CDC13, 400 MHz) delta ppm: 8.29, 7.77, 6.76 (dd, each 1H); 7.1-7.44 (m, 5H); 5.46 (t, 1H, CHPh); 3.83, 3.59 (m, each H); 2.95 (dd, 1H); 2.65-2.80 (m, 4H); 2.25 (m, 1H); 2.33 (s, 3H, NCH3). Example 2 2-(4-methyl-2-phenylpiperazin-1-yl)-3-cyanopyridine oxalate After adding 21.1 g (119.7 mmol) of <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong>, 24.0 g (173.2 mmol) of 2-chloro-3-cyanopyridine, and 16.6 g (164.1 mmol) of triethylamine to 42 g of dimethylformamide, the mixture was reacted at 125 to 130 °C for 24 hours under a nitrogen atmosphere. After distilling out triethylamine and dimethylformamide from the reactant solution under a reduced pressure, the solution was added with 32 ml of water and 87 g of ethyl acetate, and then a pH value thereof was adjusted to 8 to 9 with 10 percent aqueous sodium hydroxide solution. After phase-separating the solution, an organic layer was added with 24 g of methanol, and then 15.2 g of oxalic acid. This solution was filtrated to collect crystals, and then the crystals collected were dried to obtain 31.9 g of an objective compound (HPLC content: 92.4 percent, the yield from <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong> was 66.8 percent). Example 3 2-(4-methyl-2-phenylpiperazin-1-yl)-3-cyanopyridine oxalate In 57.3 kg of dimethylformamide solution containing 21.3 kg of <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong>, 22.2 kg of 2-chloro-3-cyanopyridine and 15.3 kg of triethylamine were added, the mixture was reacted at 114 to 125 °C for 17 hours under a nitrogen atmosphere. The reaction solution was concentrated under a reduced pressure. The distillated amount was 36 kg. The residue was added with 29.3 kg of water and then a pH value thereof was adjusted to 8.45 with 25 percent aqueous sodium hydroxide solution. This solution was added with 79.2 kg of ethyl acetate, washed with 20 kg of 5 percent sodium chloride solution, and then subjected to a phase separation. An organic layer was added with 23.1 kg of methanol, and then added with 13.9 kg of oxalic acid dihydrate at a temperature of 45 to 48 °C for about 1 hour. The solution was stirred at the temperature for 1 hour, filtrated at around 35 °C to collect crystals, and then the crystals collected were washed with a mixture of 42.2 kg of ethyl acetate and 12.4 kg of methanol. The crystals were dried at around 50 °C under a reduced pressure to obtain 32.65 kg of an objective compound (HPLC content: 90.2 percent, the yield from <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong> was 66.2 percent). |
| 56.6% |
|
Comparative Example 1 After adding 21.1 g (119.7 mmol) of <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong>, 20.0 g (144.4 mmol) of 2-chloro-3-cyanopyridine, 12.8 g (126.3 mmol) of triethylamine, and 2.0 g (12.0 mmol) of potassium iodide to 42 g of dimethylformamide, the mixture was reacted at 125 to 130 °C for 24 hours under a nitrogen atmosphere. After distilling out triethylamine and dimethylformamide from the reactant solution under a reduced pressure, the residue was added with 32 ml of water and 87 g of ethyl acetate, and then a pH value thereof was adjusted to 8 to 9 with 10 percent aqueous sodium hydroxide solution. After phase-separating the solution, an organic layer was added with 24 g of methanol, and then 15.2 g of oxalic acid. This solution was filtrated to collect crystals, and then the crystals collected were dried to obtain 26.6 g of 2-(4-methyl-2-phenylpiperazin-1-yl)-3-cyanopyridine oxalate (HPLC content: 93.8 percent, the yield from <strong>[5271-27-2]1-methyl-3-phenylpiperazine</strong> was 56.6 percent). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping