|
|
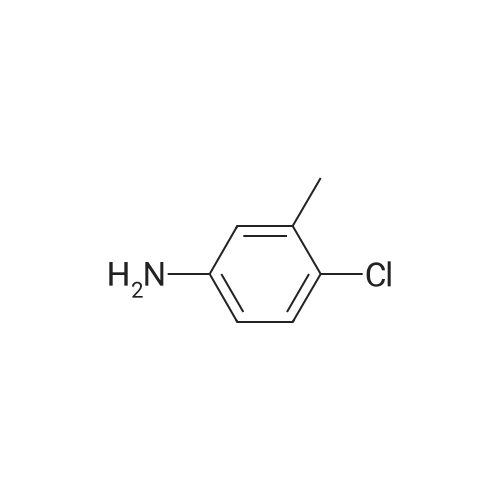
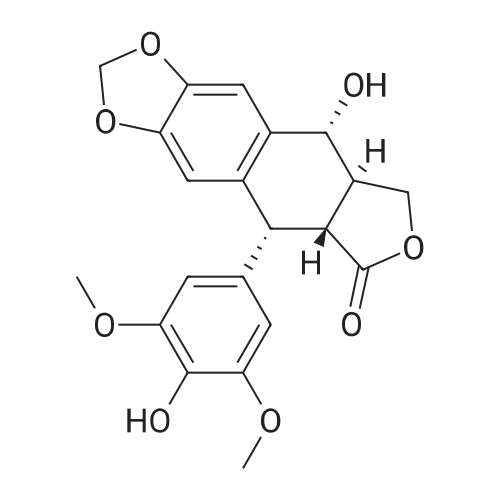
1) Synthesis of 4-N-(<strong>[7149-75-9]4-chloro-3-methylaniline</strong>)-4-deoxy-4 ?-demethylepipodophyllotoxin: taking 1 mol of activated product of position 4 of C-ring of 4?-demethylepipodophyllotoxin (prepared in preparatory test example 1), which is then dried in vacuo at 45° C. for 2 hours; under protection of nitrogen, dried dichloromethane were added into a 4-necked flask, then adding dried activated product of position 4 of C-ring of 4?-demethylepipodophyllotoxin and 2 mol of <strong>[7149-75-9]4-chloro-3-methylaniline</strong>, adding 0.36 g of BaCO3, stirring for reaction at 25° C. for 24 hours; reaction liquid is rotary dried, then obtaining crude product of 4-N-(<strong>[7149-75-9]4-chloro-3-methylaniline</strong>)-4-deoxy-4?-demethylepipodophyllotoxin. (0042) (2) Separation and purification of 4-N-(<strong>[7149-75-9]4-chloro-3-methylaniline</strong>)-4-deoxy-4?-demethylepipodophyllotoxin: Separation and purification using silica gel column chromatography and gel column chromatography: (0043) (A) using normal phase silica gel column (normal phase silica gel: China Qingdao Haiyang Chemical Co., Ltd, HG/T2354-92; separation system: Swiss Buchi isocratic fast chromatography system; chromatographic column: Swiss Buchi glass column C-690 with length of 460 mm and inner diameter of 15 mm) or a similar polar column separation; taking system of chloroform:acetone=10:1 as eluent, with sample volume of 2 ml, constant flow rate of 1.0 ml/min; each of 2 ml of eluent as a fraction were collected. Using normal phase silica gel thin layer (efficient silica gel thin layer by Merck, Germany) or thin layer with similar polarity, each of fractions are viewed; taking system of chloroform:acetone=10:1 as a developing agent, fractions with Rf value of 0.5 are merged; the sample after merged is subjected to vacuum drying, stored at 4° C. in the refrigerator under dark conditions, as samples to be purified. (B) separating by gel column chromatography (gel: Sephadex LH-20; Separation column: glass column with length 480 mm and inner diameter of 30 mm); loading processed gel Sephadex LH-20 into column by wet method to be balanced with methanol. The sample to be purified is dissolved in 6 ml of methanol, adsorbed at flow rate of 0.6 ml/min of sample and then eluted at flow rate of 0.6 ml/min with 600 ml of methanol, eluate was collected to a bottle every 10 ml, each fraction is checked with normal phase silica gel thin layer (effective silica gel thin layer by Merck, Germany) or thin layer with similar polar; adopting system with chloroform:acetone=5:1 as developing solvent, fractions with Rf value of 0.5 are combined; sample of white powder from vacuum drying is 4-N-(<strong>[7149-75-9]4-chloro-3-methylaniline</strong>)-4-deoxy-4?-demethylepipodophyllotoxin. (0045) 4-N-(<strong>[7149-75-9]4-chloro-3-methylaniline</strong>)-4-deoxy-4?-demethylepipodophyllotoxin: white powder: C28H26ClNO7; 523, 1H NMR (300 MHz, CDCl3): delta 2.303 (s, 3H, ?CH3), 2.965-3.012 (m, 1H, 2-H), 3.071 (dd, J=4.8 Hz, 1H, 3-H), 3.775 (s, 6H, 3?, 5?-OCH3), 3.950 (t, J=9.3 Hz, 1H, 11-H), 4.349 (t, J=7.8 Hz, 1H, 11-H), 4.556 (dd, J=4.8 Hz, 2H, 4-H, 1-H), 5.937 (d, J=6.6 Hz 2H, OCH2O), 6.316 (s, 3H, ArH), 6.421 (s, 1H, ArH), 6.508 (s, 1H, ArH), 6.740 (s, 1H, ArH), 7.126 (d, J=8.1 Hz, 1H, ArH)13C NMR (75 MHz, CDCl3): delta 20.614, 38.855, 42.090, 43.604, 52.836, 56.695, 69.103, 101.785, 108.152, 109.369, 110.126, 111.076, 114.935, 123.484, 130.029, 130.682, 130.816, 132.122, 134.289, 137.243, 146.356, 146.682, 147.781, 148.463, 175.120 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping