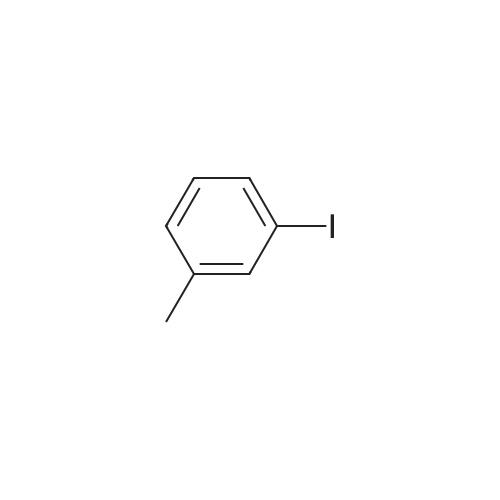
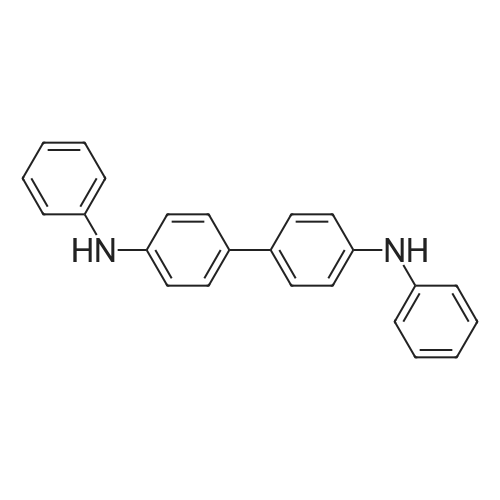
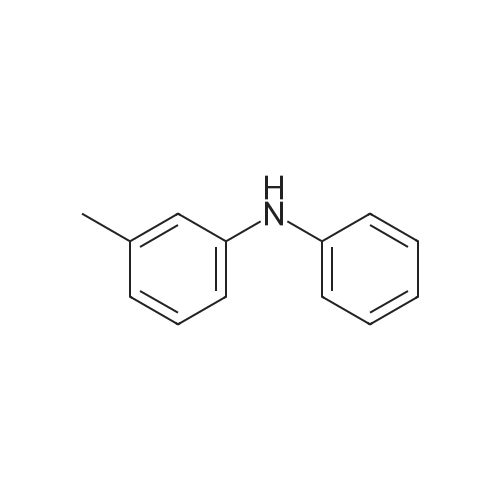
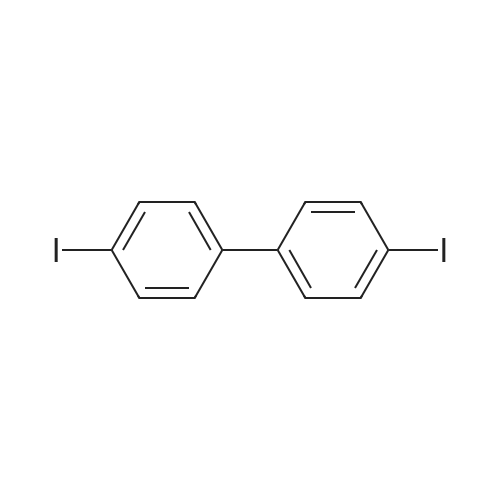
|
With potassium hydroxide;1,10-Phenanthroline; copper dichloride; In toluene; at 125℃; for 6.5h;Product distribution / selectivity; |
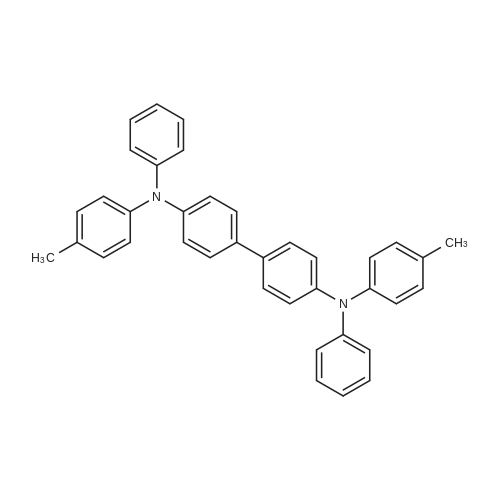
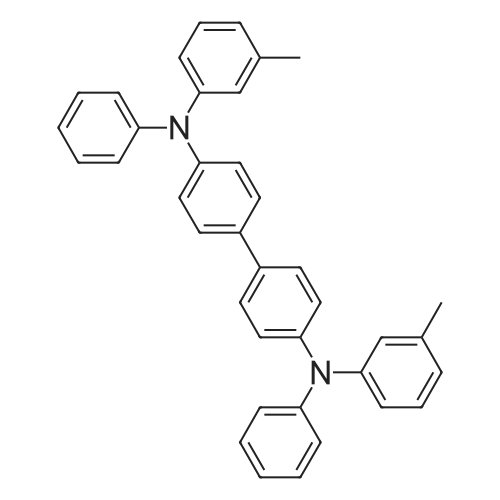
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, and a condensing tube) was communicated with a nitrogen source and contained 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 24 L of toluene, 27 g of 1,10-phenanthroline (0.14 mole), 16 g of cuprous chloride (0.14 mole) and 168 g potassium hydroxide (3 moles). The mixture solution was stirred for 30 minutes and heated to 125° C. The mixture solution was further stirred to react for 6 hours and had added thereto 500 ml of toluene, 500 ml of deionized water and 400 g of acetic acid to neutralize the potassium hydroxide. The mixture solution was held at 70° C. and then poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. a toluene solution, was removed from the mixture solution, washed with 1 L of deionized water twice and kept at 60° C. The toluene solution was filtered by 500 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 605 g of a crude mixture of benzidine compounds in the form of a white solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 500 g, has a melting point range of 169-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With potassium hydroxide;1,10-Phenanthroline; copper dichloride; In m-xylene; at 139℃; for 7.5h;Heating / reflux;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, a condensing tube, and a Dean-Stark device) was communicated with a nitrogen source and accommodated 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2.4 L of m-xylene, 27 g of 1,10-phenanthroline (0.14 mole), 16 g of cuprous chloride (0.14 mole) and 168 g potassium hydroxide (3 moles). The mixture solution was stirred for 30 minutes and heated to a reflux temperature of 139° C. The mixture solution was further stirred to react for 7 hours and had further added thereto 1 L of m-xylene, 1 L of deionized water and 400 g of acetic acid to neutralize the potassium hydroxide. The mixture solution was held at 65° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. an m-xylene solution, was removed from the mixture solution, washed with 2 L of deionized water twice and kept at 55° C. The m-xylene solution was filtered by 600 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 610 g of a crude mixture of benzidine compounds in the form of a Elite solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 585 g, has a melting point range of 169-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With potassium hydroxide;1,10-Phenanthroline; copper dichloride; In xylene; at 145℃; for 7.5h;Heating / reflux;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, a condensing tube, and a Dean-Stark device) was communicated with a nitrogen source and accommodated 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2.4 L of xylene, 27 g of 1,10-phenanthroline (0.14 mole), 16 g of cuprous chloride (0.14 mole) and 168 g of potassium hydroxide (3 moles). The mixture solution was stirred for 30 minutes and heated to a reflux temperature of 145° C. The mixture solution was further stirred to react for 7 hours and had further added thereto 1 L of o-xylene, 1 L of deionized water and 400 g of acetic acid to neutralize the potassium so hydroxide. The mixture solution was held at 100° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. an o-xylene solution, was removed from the mixture solution, washed with 1.5 L of deionized water twice and kept at 70° C. The o-xylene solution was filtered by 20 g of Alcoa-C neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 615 g of a crude mixture of benzidine compounds in the form of a white solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 600 g, has a melting point range of 168-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With potassium hydroxide; 18-crown-6 ether; copper; In m-xylene; at 139℃; for 10.5h;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, and a condensing tube) was communicated with a nitrogen source and contained 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2.4 L of m-xylene (0.14 mole), 150 g of copper powder (2.4 moles), 168 g potassium hydroxide (3 moles) and 35 g of 18-crown-6-ether. The mixture solution was stirred for 30 minutes and heated to a reflux temperature of 139° C. The mixture solution was further stirred to react for 10 hours and had further added thereto 1 L of m-xylene, 1 L of deionized water and 400 g of acetic acid to neutralize the potassium hydroxide. The mixture solution was held at 65° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. an m-xylene solution, was removed from the mixture solution, washed with 2 L of deionized water twice and kept at 55° C. The m-xylene solution was filtered by 600 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 620 g of a crude mixture of benzidine compounds in the form of a white solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 600 g, has a melting point range of 169-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With potassium hydroxide; copper; In Soltrol/170; at 190℃; for 10.5h;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, and a condensing tube) was communicated with a nitrogen source and accommodated 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2 L of Soltrol.(R)./170, 150 g of copper powder (2.4 moles) and 168 g potassium hydroxide (3 moles). The mixture solution was stirred for 30 minutes and heated to 190° C. The mixture solution was further stirred to react for 10 hours and had further added thereto 1 L of toluene, 1.5 L of deionized water and 400 g of acetic acid to neutralize the potassium hydroxide. The mixture solution was held at 65° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. a toluene solution, was removed from the mixture solution, washed with 2 L of deionized water twice and kept at 55° C. The toluene solution was filtered by 500 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 630 g of a crude mixture of benzidine compounds in the form of a white solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 600 g, has a melting point range of 167-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With sodium t-butanolate;palladium diacetate; tri-tert-butyl phosphine; In xylene; at 125℃; for 5.5h;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, a condensing tube, and a Dean-Stark device) was communicated with a nitrogen source and accommodated 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2.5 L of xylene, 0.183 g of Pd(OAc)2 (0.051 mole), 0.14 g of P(t-Bu)3 (0.043 mole) and 350 g NaO-(t-Bu) (3.64 mole). The mixture solution was stirred for 30 minutes and heated to a reflux temperature of 125° C. The mixture solution was further stirred to react for 5 hours and had further added thereto 500 ml of o-xylene and 500 ml of deionized water. The mixture solution was held at 65° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. an o-xylene solution, was removed from the mixture solution, washed with 1 L of deionized water twice and kept at 55° C. The o-xylene solution was filtered by 500 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 660 g of a crude mixture of benzidine compounds in the form of a white solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 580 g, has a melting point range of 168-170° C., and is a final mixture of the benzidine compounds having high purity. |
|
With sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0); In toluene; at 110℃; for 5.5h;Product distribution / selectivity; |
A 5 L tri-neck round bottom flask (equipped with a mechanical stirrer, a thermal controller, a condensing tube, and a Dean-Stark device) was communicated with a nitrogen source and accommodated 500 g of dibromobiphenyl (1.6 moles), 620 g of 3-methyldiphenylamine (3.4 moles), 50 g of diphenylamine (0.3 mole), 2.4 L of toluene, 7.36 g of Pd2(dba)3 (0.008 mole) (prepared according to J. Org. Chem. 2000,65,p.5330) and 350 g NaO-(t-Bu) (3.64 mole). The mixture solution was stirred for 30 minutes and heated to a reflux temperature of 110° C. The mixture solution was further stirred to react for 5 hours and had further added thereto 500 ml of toluene and 500 ml of deionized water. The mixture solution was held at 55° C. and poured into an extracting bottle to place for 10 minutes until layers of the mixture solution separated. An organic layer, i.e. a toluene solution, was removed from the mixture solution, washed with 2 L of deionized water twice and kept at 55° C. The toluene solution was filtered by using 500 g of Woelm neutral alumina to obtain a filtering liquid. Then, the filtering liquid was dried to obtain 650 g of a crude mixture of benzidine compounds in the form of a yellow solid. Lastly, n-octane was used to dissolve the crude mixture and to re-crystallize the benzidine compounds in the form of pure white crystal. The pure white crystal weighed 550 g, has a melting point range of 169-170° C., and is a final mixture of the benzidine compounds having high purity. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping