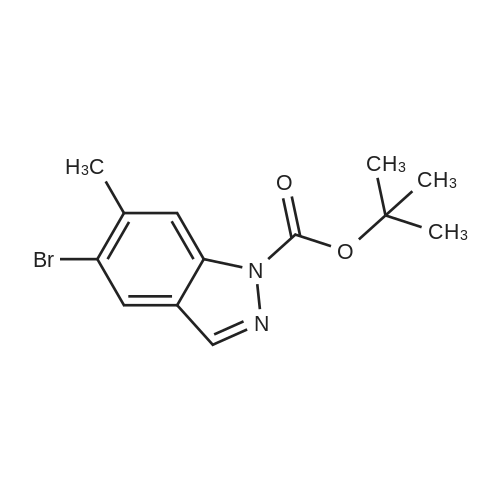
| 47% |
With tetrakis(triphenylphosphine) palladium(0); water; sodium carbonate; In 1,4-dioxane; at 100℃; for 18h;Inert atmosphere; |
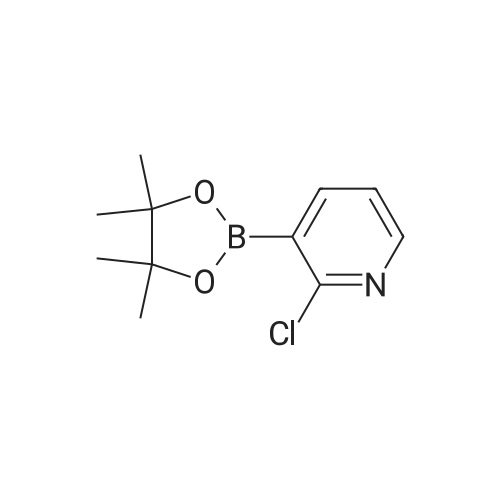
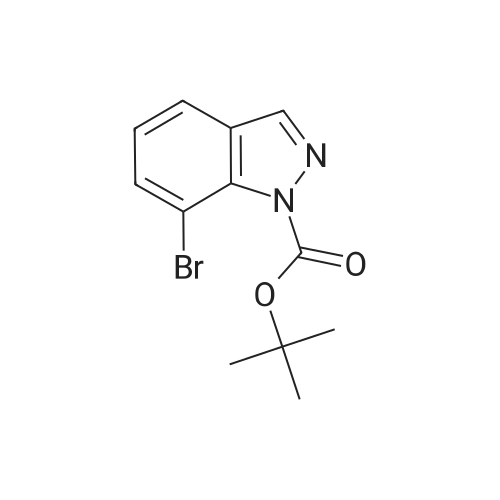
[0150j A single necked round bottom flask (250 mL) equipped with a magnetic stir bar was charged with tert-butyl 5-bromo-1H-indazole-carboxylate (4.0 g, 13.4 mmol) dissolved in 1,4- dioxane (130 mL), 2-chloro-3-pyridine boronic acid pinacol ester (4 g, 16.7 mmol), Pd(PPh3)4 (1.5 g, 1.3 mmol) and 2M aq. Na2CO3 (20 mL, 40 mmol) under nitrogen atmosphere. The rubber septum was replaced with reflux condenser containing three-way stopcock equipped with argon filled balloon. The reaction contents were stirred and air was removed from the closed reaction system by vacuum and back filled with argon. Following three cycles of degassing, the reaction mixture was heated at 100 C (oil-bath) under argon. Inflated argon balloon was emptied, refilled with argon and remounted in the course of reaction. The initial pale yellow heterogeneous reaction mixture turned to clear biphasic off-brown solution. After 18 h with no additional change in the proportion of the product (62%) as analyzed by LC/MS, the reaction mixture was cooled to room temperature. Upon concentration of the reaction mixture, EtOAc/water (200 mL / 75 mL) was transferred to the concentrate and stirred for 30 mm. The organic layer was separated and the aqueous layer extracted with EtOAc (100 mL X 2). Mg504 (20 g) and Celite (20 g) were added to combined organic layers and the contents suction filtered after stirring for 1 h. The filter cake was washed with EtOAc (300 mL) and the combined filtrates concentrated by rotary evaporator under vacuum. The crude concentrate was dissolved in in 1% MeOH/CH2C12 and absorbed on silica gel (20 g) by evaporating the solvent followed by drying. Subsequent purification by flash silica gel columnpurification of the dry powder (Combiflash companion system with RediSep silica gel column 120 g, 30-70%EtOAC/hexanes eluting solvent) provided 5-(2-chloropyridin-3-yl)-1H-indazole (1.5 g, 47%) as a white crystalline solid after concentration of the desired product fractions. 1H NMR (DMSO-d6): 13.2 (s, 1H), 8.41 (dd, 1H, J = 1.8 and 4.7 Hz), 8.13 (s, 1H), 7.90 (dd, 1H, J = 1.7 and 4.7 Hz), 7.84 (s, 1H), 7.62 (d, 1H, J = 8.8 Hz), 7.51 (dd, 1H, J = 4.7 and 7.3 Hz), 7.42 (dd, 1H, J = 1.4 and 8.5 Hz). LCMS: 95 %, MS (mle) 230 (MH+). |
| 47% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In 1,4-dioxane; water; at 100℃; for 18h;Inert atmosphere; |
A single necked round bottom flask containing a with a magnetic stir bar was charged with 5-bromo-1H-indazole (3.0 g, 15.2 mmol), di-tert-butyl dicarbonate (4.2 g, 19.2 mmol) and acetonitrile (30 mL) under mild stream of nitrogen at room temperature. Triethylamine (1.8 g, 2.5 mL, 17.7 mmol) was added in one portion to above stirred homogeneous solution followed by 4-(dimethylamino)pyridine (2.2 g, 18 mmol) over a period of 15 mm in portions. The homogenous off-brown clear reaction mixture was stirred at room temperature under nitrogen and the progress of reaction monitored by TLC (50% EtOAc/hexanes). Stirring was discontinued after 3h and the reaction mixture concentrated by rotary evaporator under vacuum. A clear viscous liquid was obtained and dissolved in EtOAc/hexanes (7:3, 200 mL), and diluted with water (75 mL). Organic layer was separated and the aqueous layer extracted with EtOAc/hexanes (1:1, 125 mL). The combined organic layers were washed with water (100 mL) followed by iN aq. HC1 (2 x 75mL) to remove 4-(dimethylamino)pyridine. The combined organic layers were washed with water (2 X 75 mL), saturated aq. NaHCO3 (2 X 75 mL) and saturated aqueous NaC1. Separated organic layers were dried over anhydrous Mg504, filtered, concentrated and dried under vacuum to provide tert-butyl 5-bromo-1H-indazole-carboxylate (4.5 g, purity 97%) as a pale yellow viscous liquid which was used without further purification. ?H NMR (DMSOd6): oe 8.36 (d, J 0.8 Hz, 1H), 8.11 (app d, J= 0.8 Hz, 1H), 8.00 (d, J= 8.8 Hz, 1H), 7.71 (app dd, J= 8.8, 0.8 Hz, 1H), 1.62 (s, 9H). LCMS: 97 %, MS (m/e) 241 (MHttBu). A single necked round bottom flask (250 mL) equipped with a magnetic stir bar was charged with tertbutyl 5-bromo-1H-indazole-carboxylate (4.0 g, 13.4 mmol) dissolved in 1,4-dioxane (130 mL), 2-chloro-3-pyridine boronic acid pinacol ester (4 g, 16.7 mmol), Pd(PPh3)4 (1.5 g, 1.3 mmol) and 2M aq.Na2CO3 (20 mL, 40 mmol) under nitrogen atmosphere. The rubber septum was replaced with reflux condenser containing three-way stopcock equipped with argon filled balloon. The reaction contents were stirred and air was removed from the closed reaction system by vacuum and back filled with argon. Following three cycles of degassing, the reaction mixture was heated at 100 C (oil-bath) under argon. Inflated argon balloon was emptied, refilled with argon and remounted in the course of reaction. The initial pale yellow heterogeneous reaction mixture turned to clear biphasic off-brown solution. After 18 h with no additional change in the proportion of the product (62%) as analyzed by LC/MS, the reaction mixture was cooled to room temperature. Upon concentration of the reaction mixture, EtOAc/water (200 mL / 75 mL) was transferred to the concentrate and stirred for 30 mm. The organic layer was separated and the aqueous layer extracted with EtOAc (100 mL X 2). Mg504 (20 g) and Celite (20 g) were added to combined organic layers and the contents suction filtered after stirring for 1 h. The filter cake was washed with EtOAc (300 mL) and the combined filtrates concentrated by rotary evaporator under vacuum. The crude concentrate was dissolved in in 1% MeOH/CH2C12 and absorbed on silica gel (20 g) by evaporating the solvent followed by drying. Subsequent purification by flash silica gel column purification of the dry powder (Combiflash companion system with RediSep silica gel column 120 g, 30-70% EtOAc/hexanes eluting solvent) provided 5-(2- chloropyridin-3-yl)-1H-indazole (1.5 g, 47%) as a white crystalline solid after concentration of the desired product fractions. ?H NMR (DMSO-d6): oe 13.2 (s, 1H), 8.41 (dd, J= 1.8 and 4.7 Hz, 1H), 8.13 (s, 1H), 7.90 (dd, J 4.7, 1.7 Hz, 1H), 7.84 (s, 1H), 7.62 (d, J= 8.8 Hz, 1H), 7.51 (dd, J= 7.3, 4.7 Hz, 1H), 7.42 (dd, J= 8.5, 1.4 Hz, 1H). LCMS: 95 %, MS (m/e)230 (MI-i?). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping