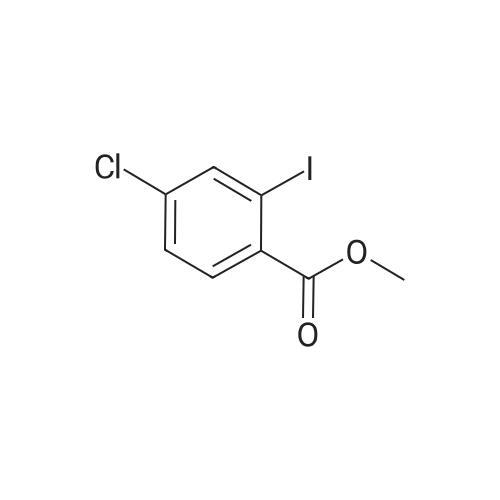
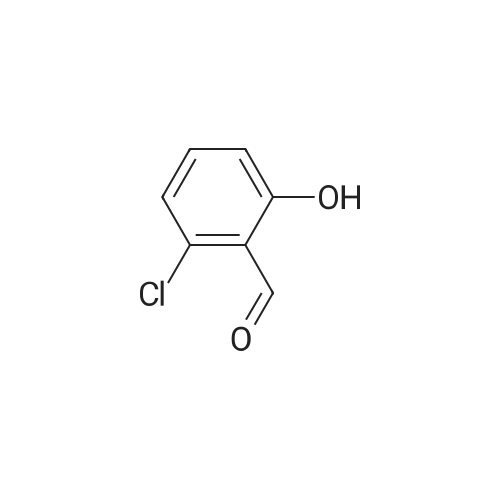
| 68% |
With water; potassium hydroxide; In acetonitrile; at 0℃; |
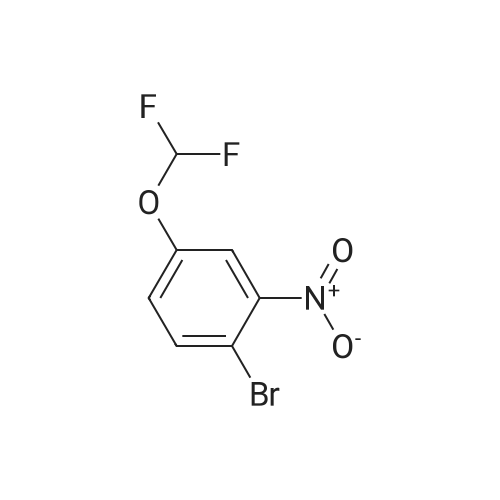
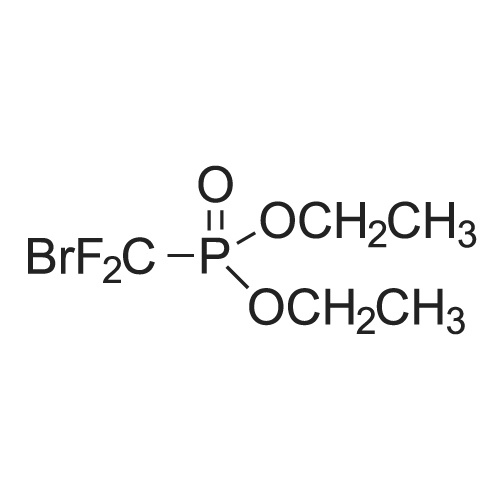
To a 250 mL round-bottom flask was added <strong>[18362-30-6]2-chloro-6-hydroxybenzaldehyde</strong> 15a (6 g, 38.32 mmol, 1.0 equiv.) and CH3CN (200 mL), followed by the dropwise addition of potassium hydroxide (21.54 g, 383,89 rnmol, 10.0 equiv.) at 0 C. Water (20 mL) was then added, followed by the dropwise addition of diethyl (bromodifluoromethyl)phosphonate (16.38 g, 61.35 mmol, 1.6 equiv.) and the reaction mixture was stirred 30 min at 0 C. The resulting mixture was quenched with H2O (100 mL) and extracted with ethyl acetate (300 mL x 2). The combined organic layers were washed with brine (300 mL x 2), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. The crude product (10 mL) was purified by Flash-Prep- HPLC using the following conditions: Column, silica gel; mobile phase, eiuting with PE:EtOAc = 100:0 to 87: 13 over 14 min; Detector, UV 254 nm to provide 2-chloro-6- (difluoromethoxy)benzaldehyde 15b (5.4 g, 68%) as a yellow oil. |
| 53% |
|
To <strong>[18362-30-6]2-chloro-6-hydroxy-benzaldehyde</strong> (20 g, 128.2 mmol) in MeCN (150 mL) was added an aqueous solution of potassium hydroxide (71.7 g, 1282 mmol) in water (50 mL) at 0C and the reaction mixture was stirred at 0C for 10 minutes. Diethyl (bromodifluoromethyl) phosphonate (36.4 mL, 205.1 mmol) was added at 0C. The reaction mixture was stirred at 0C for 30 minutes. After completion of reaction (monitored by TLC), the reaction mixture was poured into water (500 mL). The aqueous layer was extracted with ethyl acetate (1 L X 2). The organic layer was washed with water (500 mL), brine (500 mL) and dried over anhydrous sodium sulphate. The organic layer was evaporated underreduced pressure to yield the crude product which was purified by column chromatography (Si02, 5% EtOAc in hexane) yielding the title compound (13.9g, 53%) as a yellow oil. 1H NMR (400 MHz, CDC13) oe 10.46 (s, 1H), 7.49 (t, J8.2 Hz, 1H), 7.37 (dd, J 8.1, 1.1 Hz, 1H), 7.20 (m, 1H), 6.61 (t, 1H). |
| 53% |
|
To <strong>[18362-30-6]2-chloro-6-hydroxy-benzaldehyde</strong> (20 g, 128.2 mmol) in MeCN (150 mL) was added an aqueous solution of potassium hydroxide (71.7 g, 1282 mmol) in water (50 mL) at 0C and the reaction mixture was stirred at 0C for 10 mi Diethyl (bromodifluoro methyl) phosphonate (36.4 mL, 205.1 mmol) was added at 0C. The reaction mixture was stirred at 0C for 30 mm. After completion of reaction (monitored by TLC), the reaction mixturewas poured into water (500 mL). The aqueous layer was extracted with ethyl acetate (1 L X 2). The organic layer was washed with water (500 mL), brine (500 mL) and dried over anhydrous sodium sulphate. The organic layer was evaporated under reduced pressure to yield the crude product which was purified by column chromatography (Si02, 5% EtOAc in hexane) yielding the title compound (13.9g, 53% yield) as a yellow oil.1H NMR (400 MHz, CDC13) oe 10.46 (s, 1H), 7.49 (t, J 8.2 Hz, 1H), 7.37 (dd, J 8.1, 1.1 Hz, 1H), 7.20 (m, 1H), 6.61 (t, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping