| 45.99% |
|
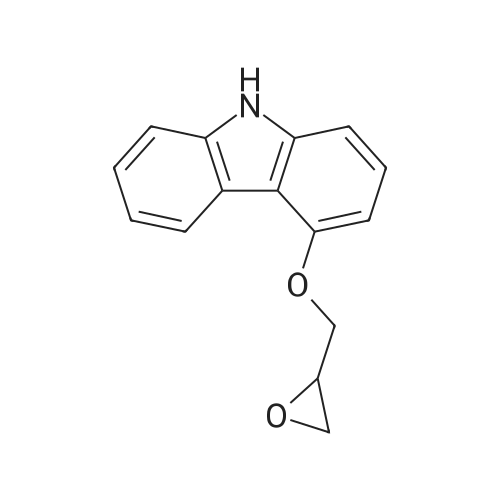
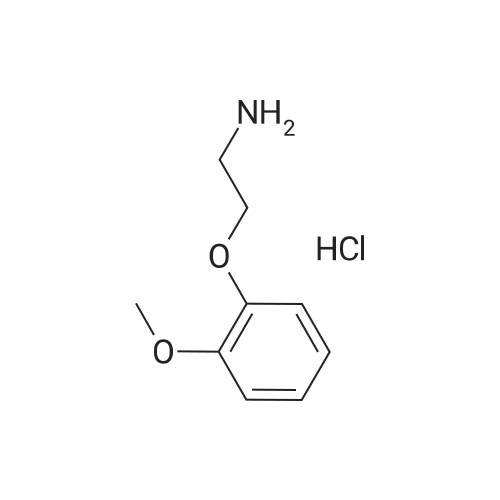
To a solution of 94.Og (0.461 moles) of 2-(2-methoxyphenoxy)-ethylamine hydrochloride in 800.0ml water is added sodium hydroxide (pellets or flakes) at room temperature till the pH of the solution is 9 to 9.5, when the solution became clear. To this clear solution is added 100.Og (0.418 moles) of 4- (2,3-epoxypropoxy) all at once. The reaction mixture is then heated at 80-85C for 45 to 60 minutes when it is monitored by thin layer chromatography, and the TLC showed completion of reaction. The reaction mixture is worked up by the addition of 500.0ml of ethyl acetate and stirring the mixture for 15 min, the ethyl acetate layer is separated, dried over sodium sulphate and evaporated to dryness to obtain Carvedilol, which is recrystallized from ethyl acetate. Yield: 78.0g (45.99%) M. P: 114 C (lit M. P. 113 to 116 C, Merck index 13th edition) 1H NMR (200 MHZ, in CDCI3) 8 (ppm) 8.2 (bs,lH, exchanges with d2O), 6.8-8.3 (m 7H) 4.2-4.0 (m 1H), 3.8 (s 3H), 3-3.2 (t 4H), 2.8 (m 4H), 1.9 (bs,lH, exchanges with d20) |
| 43.05% |
|
To a solution of 94.Og (0.461 moles) of 2-(2-methoxyphenoxy)-ethylamine hydrochloride in 400.0ml water and 400ml isopropyl alcohol is added sodium hydroxide (pellets or flakes) at room temperature till the pH of the solution was 9 to 9.5, when the solution became clear. To this clear solution is added 100.Og (0.418 moles) of 4- (2,3-epoxypropoxy) all at once. The reaction mixture is then refluxed for 4 to 5 hours when it is monitored by thin layer chromatography, and the TLC showed completion of reaction. The reaction mixture is worked up by the addition of 500.0ml of ethyl acetate and stirring the mixture for 15 min, the ethyl acetate layer is separated, dried over sodium sulphate and evaporated to dryness to obtain Carvedilol, which is recrystallized from ethyl acetate. Yield: 73.Og (43.05%) M. P: 114 (lit M. P. 113 to 116 C, Merck index 13th edition) 1H NMR (200 MHZ, in CDC13) No. (ppm) 8.2 (bs,lH, exchanges with d20), 6.8-8.3 (m 7H) 4.2-4.0 (m 1H), 3.8 (s 3H), 3-3.2 (t 4H), 2.8 (m 4H), 1.9 (bs,lH, exchanges with d20) |
| 41% |
With potassium carbonate; In i-Amyl alcohol; at 80 - 85℃; for 7h; |
To a mixture of 245.2 G of anhydrous potassium carbonate and 374.5 g of 2- (2- methoxyphenoxy) ethylamin hydrogenchloride monohydrate (IV) in 1000 ml of isoamyl alcohol, stirred at the temperature of 80 C, are added, in four portions during 5 hours, a total of 202.1 g of 4-(OXIRANE-2-YLMETHOXY)-9H-CARBAZOLE (II). After adding the whole amount the reaction mixture is stirred for two further hours at a temperature of 80 to 85 C. When the epoxide has reacted the mixed salts are filtered off from the reaction mixture and isoamyl alcohol is distilled off from the filtrate. A honey-like concentrate is, when hot, dissolved in 1000 ml of ETHYLACETATE, the solution is cooled to the temperature of 30 C, inoculated, and stirred for 30 minutes. After the crystal precipitates, the mixture is cooled to 0 C and stirred for 5 hours. The CRYSTALLISED crude Carvedilol is filtered off and washed with cooled ethylacetate. The wet crude Carvedilol is dissolved while hot in 1000 mi of ethylacetate, activated carbon is added and the mixture is stirred for a further 30 minutes. Then the mixture is filtered through diatomaceous earth and the filter is washed with 500 ml of hot ethylacetate. The filtrate is cooled to the temperature of 45 C and stirred for 30 minutes, then it is cooled down to the temperature of 5 C and stirred for 4 hours. The crystallised, purified CARVEDILOL is filtered off, washed with cooled ethylacetate and dried. The obtained, purified Carvedilol is RECRYSTALLISED from 1000 ML ethylacetate, the CRYSTALLISED CARVEDILOL substance is centrifuged, washed with cooled ethylacetate and dried at the temperature of 40 C in a vacuum drier, product being obtained in 41 % YIELD. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping