|
With hydrogenchloride; In diethyl ether; water; |
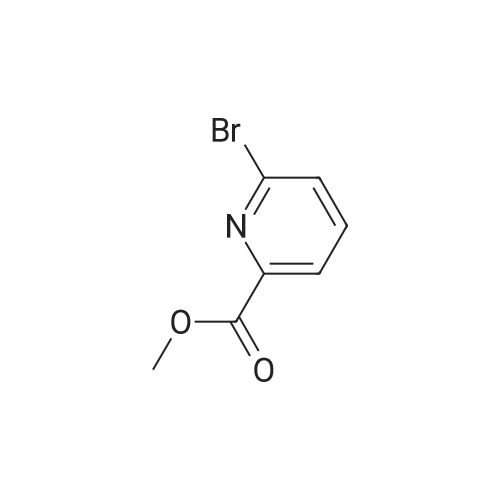
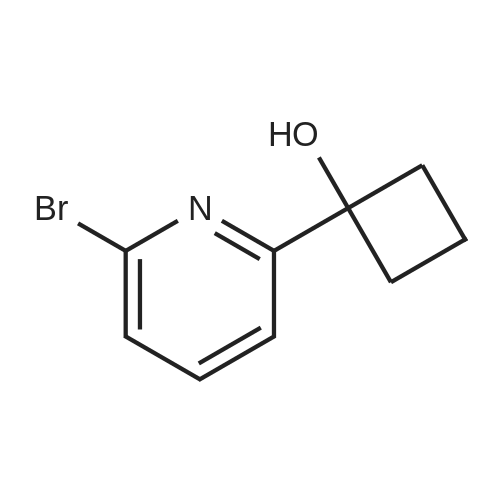
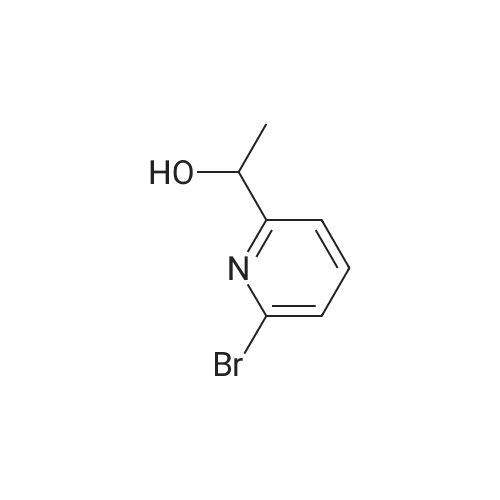
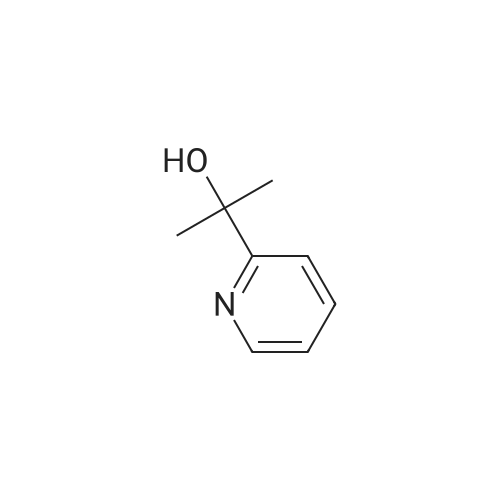
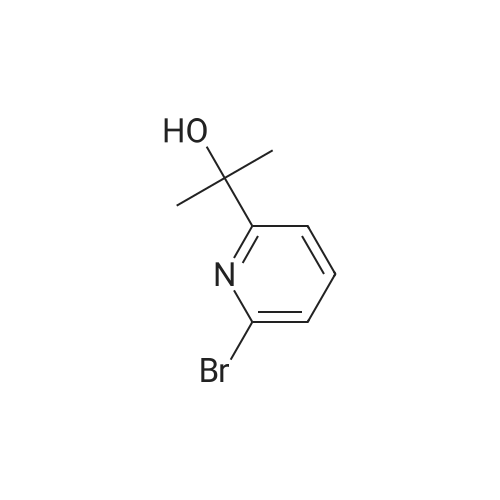
1) Production of 2-(6-bromo-2-pyridinyl)-2-propanol In a nitrogen atmosphere, 30 mL of 3 M methylmagnesium iodide/diethyl ether was added to 300 mL of diethyl ether solution of 8.72 g of methyl 6-bromopyridine-2-carboxylate. Water and 2 N hydrochloric acid were added to the reaction liquid, and extracted with ethyl acetate. This was washed with aqueous saturated sodium hydrogencarbonate solution and saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure to obtain 8.51 g of crude 2-(6-bromo-2-pyridinyl)-2-propanol as a yellow oily substance. 1H-NMR (400 MHz, CDCl3) delta: 7.56 (1H, t, J=7.8 Hz), 7.38 (1H, dd, J=7.8, 1.0 Hz), 7.36 (1H, dd, J=7.8, 1.0 Hz), 1.55(6H, s). ESI-MS Found: m/z[M+H]+ 216, 218. |
|
|
Reference Example 2:Production of 2-allyl-l -[6-(I -hydroxy- 1 -methyl ethyl)pyridin-2-yl] -6-{ [4-(4-methylpiperazin- 1- yl)phenyl]ammo>-l,2-dihvdro-3H-pyrazolo[3,4-dlpyrimidin-3-one1) Production of 2-(6-bromo-2-pyridinyl)-2-propanol:In a nitrogen atmosphere, 30 mL of 3 M methylmagnesium iodide/diethyl ether was added to 300 mL of diethyl ether solution of 8.72 g of methyl 6-bromopyridine-2- carboxylate. Water and 2 N hydrochloric acid were added to the reaction liquid, and extracted with ethyl acetate. This was washed with aqueous saturated sodium hydrogencarbonate solution and saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure to obtain 8.51 g of crude 2-(6-bromo-2-pyridinyl)-2- propanol as a yellow oily substance. iH-NMR (400 MHz, CDC13) 6: 7.56 (IH, t, J=7.8 Hz), 7.38 (IH, dd, J=7.8, 1.0 Hz), 7.36 (IH, dd, J=7.8, 1.0 Hz), 1.55(6H, s). ESI-MS Found: m/z[M+H]+ 216, 218. |
|
|
In a nitrogen atmosphere, 30 mL of 3 M methylmagnesium iodide/diethyl ether was added to 300 mL of diethyl ether solution of 8.72 g of methyl 6-bromopyridine-2- carboxylate. Water and 2N hydrochloric acid were added to the reaction liquid, and extracted with ethyl acetate. This was washed with aqueous saturated sodium hydrogencarbonate solution and saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure to obtain 8.51 g of crude 2-(6-bromo-2-pyridinyl)-2- propanol as a yellow oily substance. iH-NMR (400 MHz, CDC13) delta: 7.56 (IH, t, J=7.8 Hz), 7.38 (IH, dd, J=7.8, 1.0 Hz), 7.36 (IH, dd, J=7.8, 1.0 Hz), 1.55(6H, s). ESI-MS Found: m/z[M+H]+ 216, 218. |
|
With hydrogenchloride; In diethyl ether; water;Inert atmosphere; |
Step 1) Production of 2-(6-bromo-2-pyridinyl)-2-propanol: In a nitrogen atmosphere, 30 mL of 3 M methylmagnesium iodide/diethyl ether was added to 300 mL of diethyl ether solution of 8.72 g of methyl 6-bromopyridine-2- carboxylate. Water and 2 N hydrochloric acid were added to the reaction liquid, and extracted with ethyl acetate. This was washed with aqueous saturated sodium hydrogencarbonate solution and saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure to obtain 8.51 g of crude 2-(6-bromo-2-pyridinyl)-2- propanol as a yellow oily substance. iH-NMR (400 MHz, CDC13) delta: 7.56 (1H, t, J=7.8 Hz), 7.38 (1H, dd, J=7.8, 1.0 Hz), 7.36 (1H, dd, J=7.8, 1.0 Hz), 1.55(6H, s). ESI-MS Found: m/z[M+H]+ 216, 218. |
|
In diethyl ether;Inert atmosphere; |
In a nitrogen atmosphere, 30 mL of 3 M methylmagnesium iodide/diethyl ether was added to 300 mL of diethyl ether solution of 8.72 g of methyl 6-bromopyridine-2-carboxylate. Water and 2 N hydrochloric acid were added to the reaction liquid, and extracted with ethyl acetate. This was washed with aqueous saturated sodium hydrogencarbonate solution and saturated saline water, and dried with anhydrous magnesium sulfate. The solvent was evaporated away under reduced pressure to obtain crude 2-(6-bromo-2-pyridinyl)-2-propanol as a yellow oily substance. 1H-NMR (400 MHz, CDCl3) delta: 7.56 (1H, t, J=7.8 Hz), 7.38 (1H, dd, J=7.8, 1.0 Hz), 7.36 (1H, dd, J=7.8, 1.0 Hz), 1.55 (6H, s). ESI-MS Found: m/z[M+H]+ 216, 218. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping