| 70% |
Stage #1: With ammonia In ethanol at 90℃; for 24 h;
Stage #2: With hydrogenchloride In 1,4-dioxane at 20℃; for 0.5 h; |
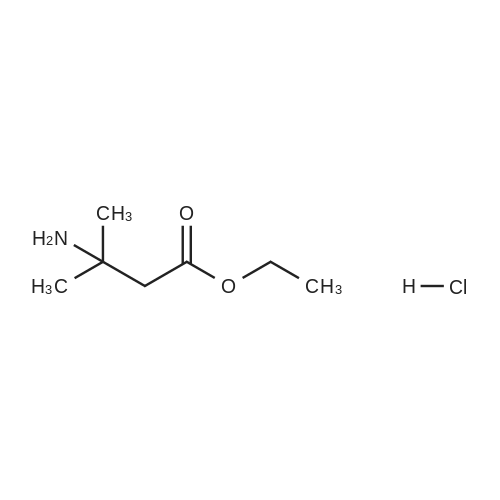
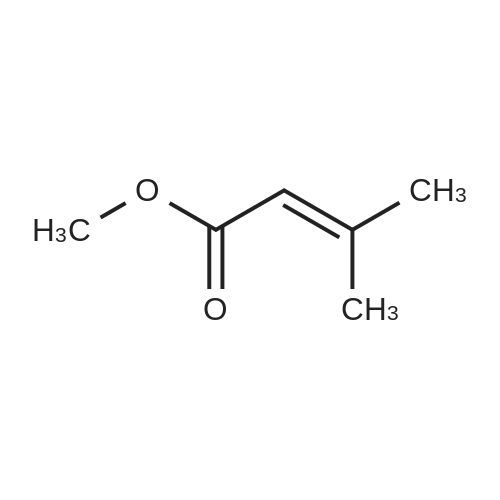
INTERMEDIATE 49Ethyl 3-ammo-3-methylbutanoate hydrochloride; To a stirred solution of ethyl 3,3-dimethylacrylate (5.0 g, 39.1 mmol) in EtOH (20 mL) in a Parr.(R). reactor at O0C was added liquid NH3 (approximately 20 mL). The reactor was sealed and heated to 9O0C for 24 h. The reaction mixture was then cooled to r.t., bubbled with nitrogen to remove the residual NH3 and treated with 4M HCl in 1 ,4- dioxane (10 mL). The reaction mixture was stirred for 30 minutes at r.t. and then evaporated in vacuo to dryness. The resulting grey paste was triturated with DCM, filtered and dried to give the title compound (5.0 g, 70percent) as a grey solid that was used without further purification. δH (CDCl3) 8.27 (3H, br. s), 4.10 (2H, q, J7.1 Hz), 2.65 (2H, s), 1.26 (6H, s), 1.20 (3H, t, J 7.1 Hz). |
| 70% |
Stage #1: With ammonia In ethanol at 0 - 90℃; for 24 h;
Stage #2: With hydrogenchloride In ethanol at 20℃; for 0.5 h; |
INTERMEDIATE 1Ethyl 3-amino-3-methylbutanoate hydrochlorideTo a stirred solution of ethyl 3,3-dimethylacrylate (5.0 g, 39.1 mmol) in EtOH (20 mL) in a Parr.(R). reactor at 00C was added liquid NH3 (ca 20 mL). The reactor was sealed and heated to 900C for 24 h. The reaction mixture was then cooled to r.t., bubbled with nitrogen to remove the residual NH3 and treated with 4M HCl in dioxane (10 mL). The reaction mixture was stirred for 30 minutes at r.t. and then evaporated in vacuo to dryness. The resulting grey paste was triturated with DCM, filtered and dried to give the title compound (5.0 g, 70percent) as a grey solid that was used without further purification. 6H (CDCl3) 8.27 (3H, br. s), 4.10 (2H, q, J7.1 Hz), 2.65 (2H, s), 1.26 (6H, s), 1.20 (3H, t, J 7.1 Hz). |
| 70% |
Stage #1: With ammonia In ethanol at 0 - 90℃; for 24 h;
Stage #2: With hydrogenchloride In 1,4-dioxane; ethanol at 20℃; for 0.5 h; |
INTERMEDIATE 1Ethyl 3-amino-3-methylbutanoate hydrochlorideTo a stirred solution of ethyl 3,3-dimethylacrylate (5.0 g, 39.1 mmol) in EtOH (20 mL) in a Parr.(R). reactor at O0C was added liquid NH3 (ca 20 mL). The reactor was sealed and heated to 9O0C for 24 h. The reaction mixture was then cooled to r.t, bubbled with nitrogen to remove the residual NH3 and treated with 4M HCl in 1 ,4-dioxane (10 mL). The reaction mixture was stirred for 30 minutes at r.t. and then evaporated in vacuo to dryness. The resulting grey paste was triturated with DCM, filtered and dried (MgSO4) to give the title compound (5.0 g, 70percent) as a grey solid that was used without further purification. δH (CDCl3) 8.27 (3H, br s), 4.10 (2H, q, J 7.1 Hz), 2.65 (2H, s), 1.26 (6H, s), 1.20 (3H, t, J7.1 Hz). |
| 70% |
Stage #1: With ammonia In ethanol at 0 - 90℃; for 24 h;
Stage #2: With hydrogenchloride In 1,4-dioxane; ethanol at 20℃; for 0.5 h; |
To a stirred solution of ethyl 3,3-dimethylacrylate (5.0 g, 39.1 mmol) in EtOH (20 mL) in a PARR.(R). reactor was added liquid NH3 (ca 20 mL) at 00C. The reactor was sealed and heated to 900C for 24 h. The reaction mixture was then cooled to r.t., bubbled with N2 to remove the residual NH3 and treated with 4M HCl in 1,4-dioxane (10 mL). The reaction mixture was stirred for 30 minutes at r.t. and then evaporated in vacuo to dryness. The resulting grey paste was triturated with DCM, filtered and dried to give the title compound as a grey solid (5.0 g, 70percent) that was used without further purification. 5H (CDCl3) 8.27 (3H, br. s), 4.10 (2H, q), 2.65 (2H, s), 1.26 (6H, s), 1.20 (3H, t). |
| 58.8% |
Stage #1: With ammonia In ethanol at -70 - 45℃; for 16 h; Autoclave
Stage #2: With hydrogenchloride In 1,4-dioxane at 0℃; for 0.5 h; Inert atmosphere |
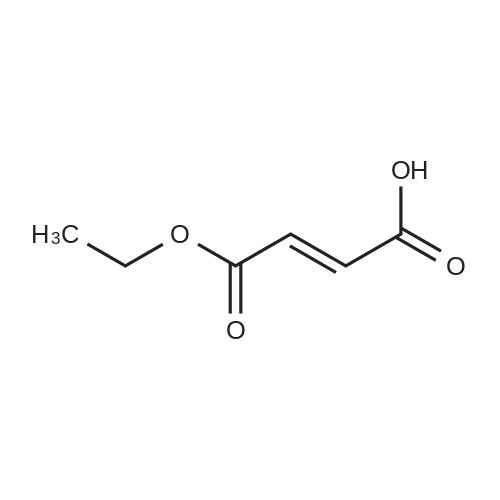
Step II-I-a: Ethyl 3-amino-3-methylbutanoate hydrochloride salt (11-I-a)To a solution of ethyl 3-methylbut-2-enoate (15 g, 117 mmol) in EtOH (40 mL) was added liquid ammonia (80 mL) at -70° C. and the reaction mixture stirred in a autoclave (200 Psi) at 45° C. for 16 h. After completion of the reaction (monitored by TLC), excess ammonia was removed by flashing N2, cooled to 0° C. and HCl in dioxane (pH-2) was added. The reaction mixture was stirred for 30 minutes at 0° C., the volatiles were removed under reduced pressure and the obtained solid was washed with diethyl ether to afford 11-I-a-HCl salt (10 g, 58.8percent) as white solid; TLC: 10percent MeOH/DCM (Rf: 0.1); 1H-NMR (DMSO d6, 200 MHz): δ 8.33 (bs, 1H), 4.09 (q, J=7.0 Hz, 2H), 2.70 (s, 2H), 1.33 (s, 6H), 1.20 (t, J=7.0 Hz, 3H); Mass: 146 [M++1]. |
| 58.8% |
With hydrogenchloride; ammonia In 1,4-dioxane; ethanol |
Step 5-I-a:
Ethyl 3-amino-3-methylbutanoate hydrochloride salt (5-I-a)
To a solution of ethyl 3-methylbut-2-enoate (15 g, 117 mmol) in EtOH (40 mL) was added liquid ammonia (80 mL) at -70° C. and the reaction mixture stirred in a autoclave (200 Psi) at 45° C. for 16 h.
After completion of the reaction (monitored by TLC), excess ammonia was removed by flashing N2, cooled to 0° C. and HCl in dioxane (pH?2) was added.
The reaction mixture was stirred for 30 minutes at 0° C., the volatiles were removed under reduced pressure and the obtained solid was washed with diethyl ether to afford 5-I-a-HCl salt (10 g, 58.8percent) as white solid; TLC: 10percent MeOH/DCM (Rf: 0.1); 1H-NMR (DMSO d6, 200 MHz): δ 8.33 (bs, 1H), 4.09 (q, J=7.0 Hz, 2H), 2.70 (s, 2H), 1.33 (s, 6H), 1.20 (t, J=7.0 Hz, 3H); Mass: 146 [M++1]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +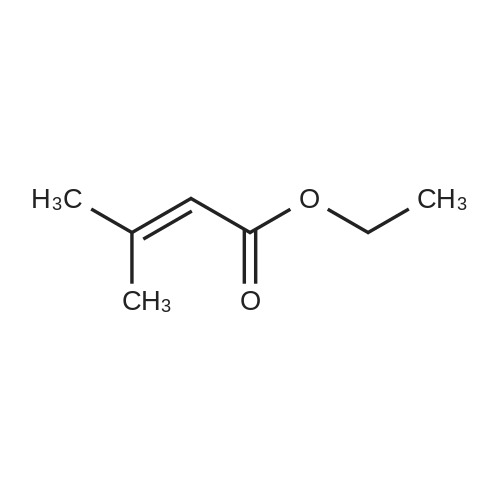

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping