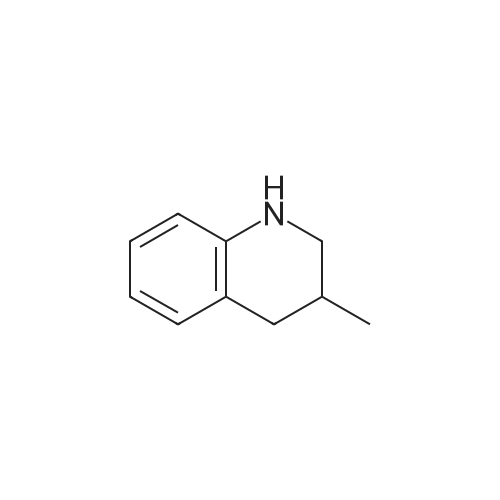
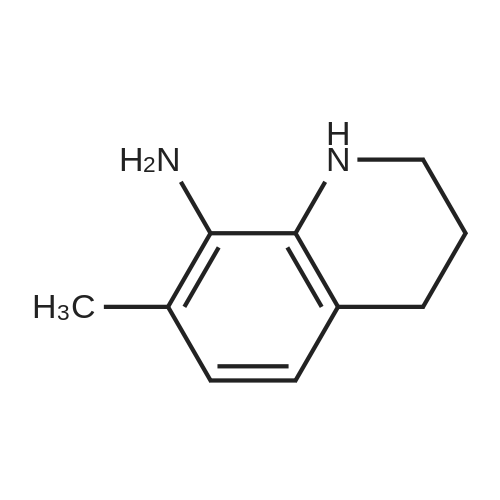
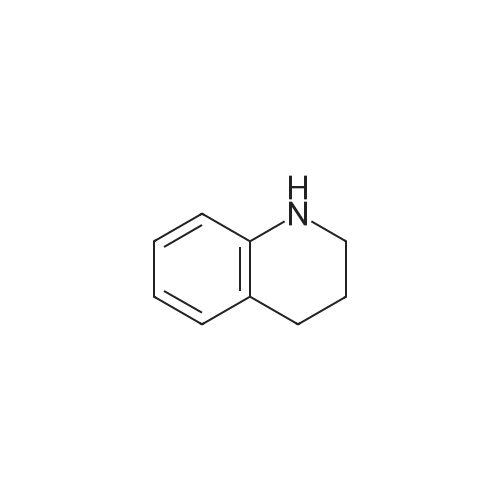
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20℃; |
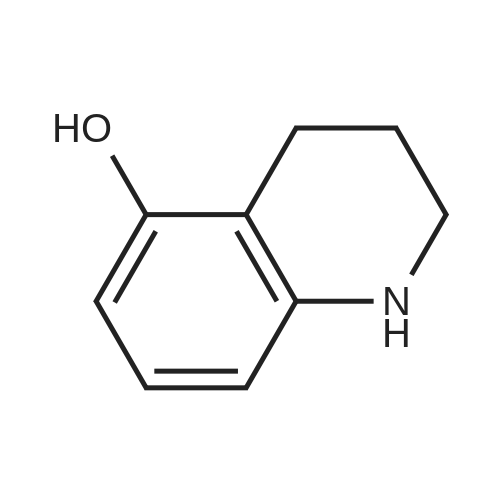
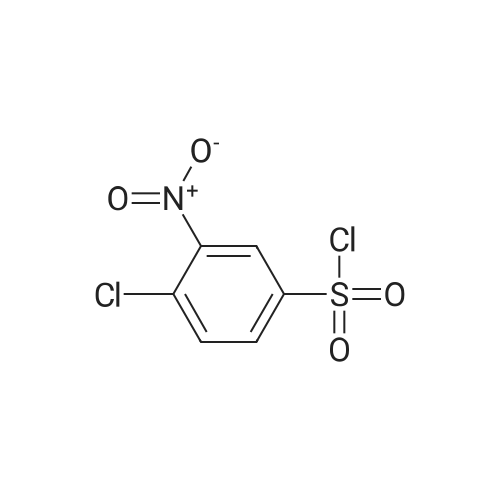
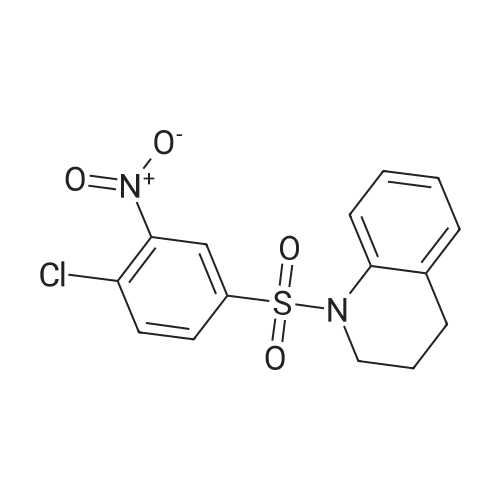
Reference Example 1 1-[(4-Chloro-3-nitrophenyl)sulfonyl]-1,2,3,4-tetrahydroquinoline A solution of 4-chloro-3-nitrobenzenesulfonyl chloride (17.0 g) in THF (50 ml) was added to 1,2,3,4-tetrahydroquinoline (9.74 g) and sodium hydrogencarbonate (8.38 g) with stirring in THF (150 ml) and water (15 ml) at room temperature, and the mixture was further stirred overnight. The reaction mixture was diluted with ethyl acetate, washed with water, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. The obtained crystals were washed with diisopropyl ether to give the object (15.7 g) as crystals. 1H-NMR (CDCl3) delta 1.66-1.78 (2H, m), 2.49 (2H, t), 3.85 (2H, t), 7.03-7.07 (1H, m), 7.14 (1H, dt), 7.23-7.27 (1H, m), 7.57 (1H, d), 7.63 (1H, dd), 7.74-7.78 (1H, m), 8.08 (1H, d). |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20℃; |
Reference Example 1; 2-Chloro-5-(3,4-dihydroquinolin-1(2H)-ylsulfonyl)aniline; To a suspension of 1,2,3,4-tetrahydroquinoline(3.12 g) and sodium hydrogen carbonate (2.66 g) in tetrahydrofuran (60 mL) were added water (6 mL) and a solution of 4-chloro-3-nitrobenzenesulfonyl chloride (5.4 g) in tetrahydrofuran (30 mL) successively, and the mixture was stirred at room temperature overnight. The reaction mixture was diluted with ethyl acetate, and the resulting mixture was washed with water, 1 mol/L hydrochloric acid, water and brine successively, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give 1-[(4-chloro-3-nitrophenyl)sulfonyl]-1,2,3,4-tetrahydroquinoline (5.0 g). This material was dissolved in tetrahydrofuran (45 mL). To the solution were added methanol (45 mL), nickel(II) bromide (0.15 g) and sodium borohydride (1.61 g) under ice-cooling, and the mixture was stirred at the same temperature for 30 minutes. Then the mixture was stirred at room temperature for 30 minutes. The reaction mixture was diluted with ethyl acetate, and the resulting mixture was washed with a saturated aqueous sodium hydrogen carbonate solution, water and brine successively, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel (eluent: n-hexane/ethyl acetate = 3/1) to give the title compound (4.33 g). |
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20℃; |
Reference Example 12 2-Chloro-5-(3,4-dihydroquinolin-1(2H)-ylsulfonyl)aniline To a suspension of 1,2,3,4-tetrahydroquinoline (3.12 g) and sodium hydrogen carbonate (2.66 g) in tetrahydrofuran (60 mL) were added water (6 mL) and a solution of 4-chloro-3-nitrobenzenesulfonyl chloride (5.4 g) in tetrahydrofuran (30 mL) successively, and the mixture was stirred at room temperature overnight. The reaction mixture was diluted with ethyl acetate, and the resulting mixture was washed with water, 1 mol/L hydrochloric acid, water and brine successively, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give 1-[(4-chloro-3-nitrophenyl)-sulfonyl]-1,2,3,4-tetrahydroquinoline (5.0 g). This material was dissolved in tetrahydrofuran (45 mL). To the solution were added methanol (45 mL), nickel(II) bromide (0.15 g) and sodium borohydride (1.61 g) under ice-cooling, and the mixture was stirred at the same temperature for 30 minutes, and then stirred at room temperature for 30 minutes. The reaction mixture was diluted with ethyl acetate, and the resulting mixture was washed with a saturated aqueous sodium hydrogen carbonate solution, water and brine successively, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel (eluent: n-hexane/ethyl acetate = 3/1) to give the title compound (4.33 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping