Alternatived Products of [ 630-25-1 ]
Product Details of [ 630-25-1 ]
| CAS No. : | 630-25-1 |
MDL No. : | MFCD00000780 |
| Formula : |
C2Br2Cl4
|
Boiling Point : |
No data available |
| Linear Structure Formula : | Cl2(Br)CC(Br)Cl2 |
InChI Key : | WJUKOGPNGRUXMG-UHFFFAOYSA-N |
| M.W : |
325.64
|
Pubchem ID : | 69426 |
| Synonyms : |
|
Application In Synthesis of [ 630-25-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 630-25-1 ]
- 1
-
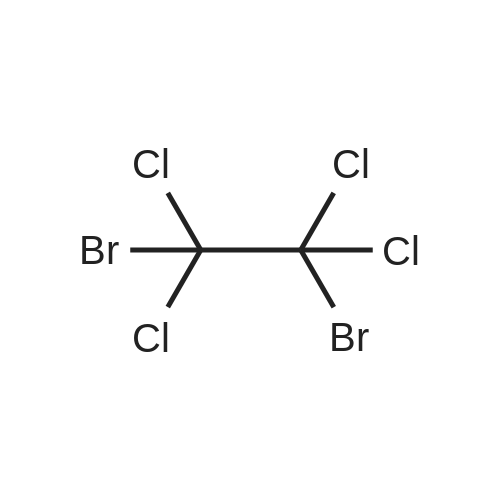 [ 630-25-1 ]
[ 630-25-1 ]

-
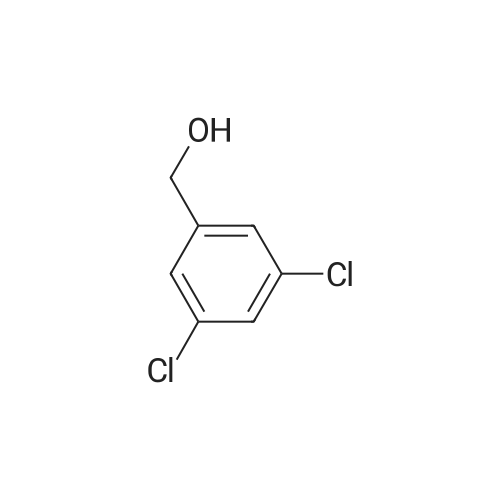 [ 60211-57-6 ]
[ 60211-57-6 ]

-
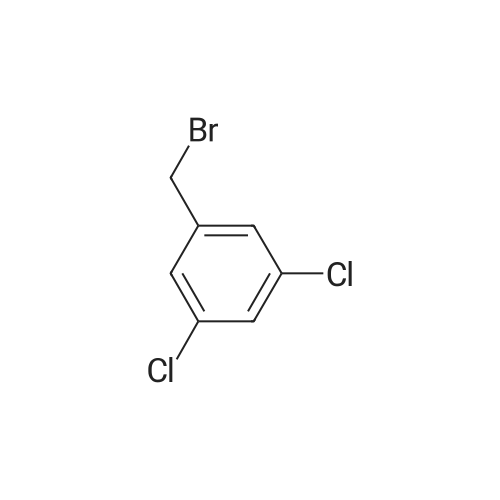 [ 7778-01-0 ]
[ 7778-01-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With triphenylphosphine; In diethyl ether; |
EXAMPLE 14 This Example illustrates the preparation of 3,5-dichlorobenzyl bromide. A solution of triphenylphosphine (8.1 g) in diethyl ether (100 cm3) was added to a stirred solution of <strong>[60211-57-6]3,5-dichlorobenzyl alcohol</strong> (5 g) and 1,2-dibromotetrachloroethane (9.9 g) in diethyl ether whilst maintaining the reaction temperature below 5 C. When the addition was complete the reaction mixture was allowed to warm to the ambient temperature (ca. 25 C.), and the mixture stirred for a further 2 hours. The precipitated solid was removed by filtration, and the filtrate concentrated by evaporation of the solvent to give 3,5-dichlorobenzyl bromide (6.8 g). 1 H nmr (CDCl3): 4.4 (s, 2H); 7.3 (s, 3H). |
|
With triphenylphosphine; In diethyl ether; |
EXAMPLE 14 This Example illustrates the preparation of 3,5-dichlorobenzyl bromide. A solution of triphenylphosphine (8.1 g) in diethyl ether (100 cm3) was added to a stirred solution of <strong>[60211-57-6]3,5-dichlorobenzyl alcohol</strong> (5 g) and 1,2-dibromotetrachloroethane (9.9 g) in diethyl ether whilst maintaining the reaction temperature below 5 C. When the addition was complete the reaction mixture was allowed to warm to the ambient temperature (ca. 25 C.), and the mixture stirred for a further 2 hours. The precipitated solid was removed by filtration, and the filtrate concentrated by evaporation of the solvent to give 3,5-dichlorobenzyl bromide (6.8 g). 1 H nmr (CDCl3): 4.4 (s,2H); 7.3 (s,3H). |
- 2
-
 [ 102-54-5 ]
[ 102-54-5 ]

-
 [ 630-25-1 ]
[ 630-25-1 ]

-
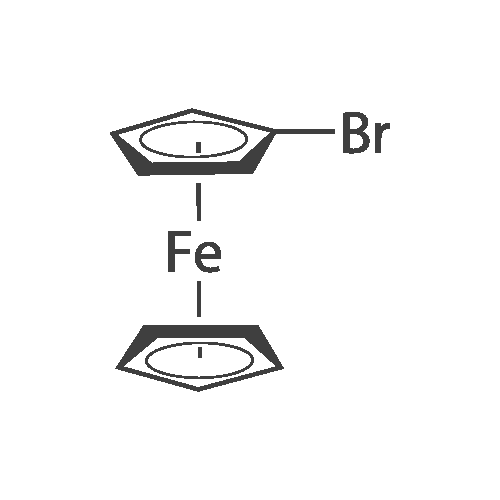 [ 1273-73-0 ]
[ 1273-73-0 ]
- 3
-
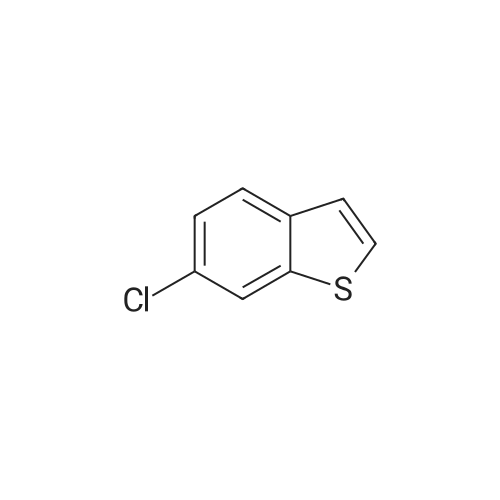 [ 66490-20-8 ]
[ 66490-20-8 ]

-
 [ 630-25-1 ]
[ 630-25-1 ]

-
 [ 1322718-91-1 ]
[ 1322718-91-1 ]
| Yield | Reaction Conditions | Operation in experiment |
| 85% |
|
6-Chlorobenzo[b]thiophene (4 g, 23.72 mmol) was dissolved in anhydrous ether (50 mL) in a 250 mL 3-neck round bottom flask with an addition funnel on top under an inert atmosphere. The resulting suspension was cooled to -78C, and sec-BuLi 1.4M (17.79 mL, 24.91 mmol) in cyclohexane was added dropwise over a period of 15 minutes. The reaction mixture was stirred for 60 minutes, keeping the temperature constant. Subsequently, 1,2-dibromo-1,1,2,2-tetrachloroethane (8.11g, 24.91g) was added portion by portion under stirring over a period of 10 minutes. The resulting mixture was slowly warmed to room temperature with stirring for another 16 hours. Subsequently, it was allowed to cool to 0C, and HCl 2N (30 mL) was added dropwise through the addition funnel and stirred for another 30 minutes. The resulting slurry was partitioned between water (100 mL) and ether (100 mL). The organics were separated, and the aqueous phase was back-extracted with ether (100 mL). The combined organic layer was dried over magnesium sulfate and the solvent was removed in vacuo to give an orange oil. The crude mixture was purified by flash chromatography using isohexane as the eluent in a standard silica solid phase to give a yellow oil (5 g, 20.20 mmol, 85%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping











