Alternatived Products of [ 6284-40-8 ]
Product Details of [ 6284-40-8 ]
| CAS No. : | 6284-40-8 |
MDL No. : | MFCD00004707 |
| Formula : |
C7H17NO5
|
Boiling Point : |
- |
| Linear Structure Formula : | CH3NHCH2(CHOH)4CH2OH |
InChI Key : | MBBZMMPHUWSWHV-BDVNFPICSA-N |
| M.W : |
195.21
|
Pubchem ID : | 8567 |
| Synonyms : |
Methylglucamin;Meglumin;Sorbitol, 1-deoxy-1-methylamino-;N-Methyl-D-glucamine;Iosulamide;1-Deoxy-1-(methylamino)-D-glucitol;Methylglucamine
|
Chemical Name : | (2R,3R,4R,5S)-6-(Methylamino)hexane-1,2,3,4,5-pentaol |
Application In Synthesis of [ 6284-40-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 6284-40-8 ]
- 1
-
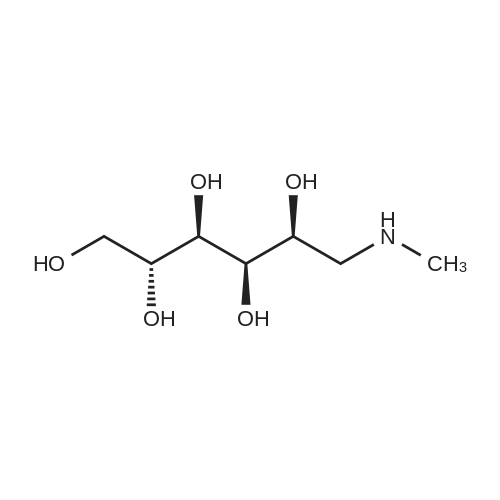 [ 6284-40-8 ]
[ 6284-40-8 ]

-
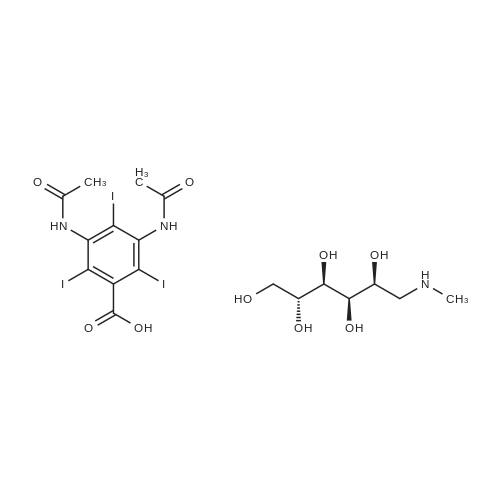
 [ 57808-65-8 ]
[ 57808-65-8 ]

-
N-methylglucamine salt of closantel
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In propylene glycol; ethanol; benzyl alcohol; |
N-methylglucamine is added to a suspension of <strong>[57808-65-8]closantel</strong> in ethanol, benzyl alcohol, and propylene glycol. With stirring, the salt of <strong>[57808-65-8]closantel</strong> forms in situ, forming a solution. Moxidectin is then added, and stirred until solution is obtained. The formulation is then brought to volume with propylene glycol. |
- 2
-
 [ 6284-40-8 ]
[ 6284-40-8 ]

-
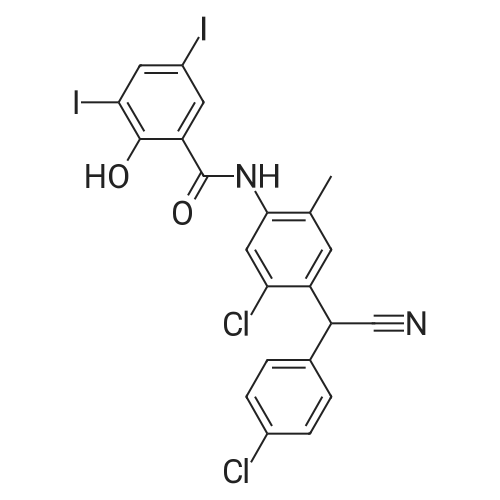
 [ 68302-57-8 ]
[ 68302-57-8 ]

-
amlexanox meglumine salt
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water; for 0.0333333h; |
The compositions of instantly soluble <strong>[68302-57-8]amlexanox</strong> formulations with meglumine are listed in the Table below. The compositions were prepared by mixing of weighted amounts of dry powders for 90 minutes in a planetary mixer. Dissolution of the formulation was tested by placing the 85 mg of powder formulation in 5 mL water. The solution becomes clear in less than 2 minutes. |
- 3
-
 [ 6284-40-8 ]
[ 6284-40-8 ]

-
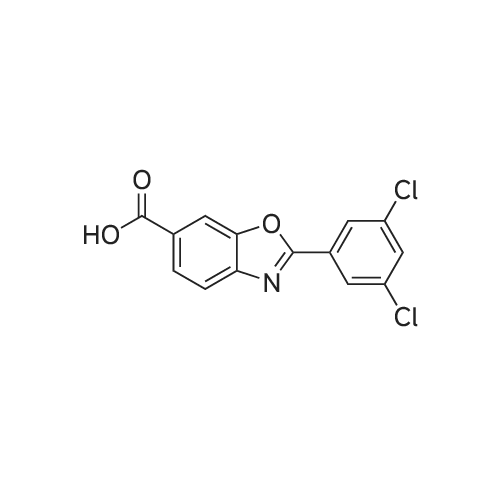 [ 594839-88-0 ]
[ 594839-88-0 ]

-
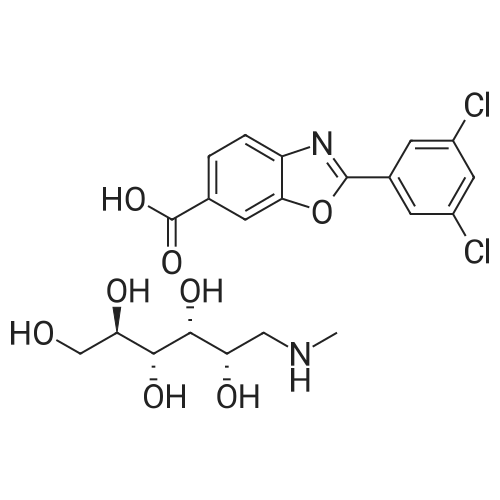 [ 951395-08-7 ]
[ 951395-08-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 82% |
In water; isopropyl alcohol; at 10 - 79℃; |
Example 1 - Preparation of Crystalline Compound 16-Carboxy-2-(3,5-dichlorophenyl)-benzoxazole free acid (2.5 g, 8.1 mmol) and 2- propanol (49 ml.) were charged to a 100 mL jacketed, 2-neck round bottom flask with magnetic stirrer. The resulting slurry was warmed to 70 C with stirring. Water (8.8 mL) was then charged. In a separate 15 mL round bottom flask a solution of N-methyl-D- glucamine (1.58 g, 8.1 mmol) in 5 mL water was prepared and dissolved with stirring. The aqueous N-methyl-D-glucamine solution was then transferred to the reaction flask over 2 min. Most (but not all) of the solids dissolved by the end of this addition. After 5 min stirring and warming to 79 C, a clear, pale yellow solution resulted. The solution was filtered through a bed of Celite, cooled to 60 C, then cooled to 10 C over 2 h. The resulting solids were collected by filtration, washing with 10 mL of 2-propanol. 3.35 g product was obtained (82% yield). |
| 82% |
In water; isopropyl alcohol; at 70 - 79℃; |
6-Carboxy-2-(3,5-dichlorophenyl)-benzoxazole free acid (2.5 g, 8.1 mmol) and 2-propanol (49 ml.) were charged to a 100 mL jacketed, 2-neck round bottom flask with magnetic stirrer. The resulting slurry was warmed to 70 C with stirring. Water (8.8 mL) was then charged. In a separate 15 mL round bottom flask a solution of N-methyl-D-glucamine (1.58 g, 8.1 mmol) in 5 mL water was prepared and dissolved with stirring. The aqueous N-methyl-D-glucamine solution was then transferred to the reaction flask over 2 min. Most (but not all) of the solids dissolved by the end of this addition. After 5 min stirring and warming to 79 C, a clear, pale yellow solution resulted. The solution was filtered through a bed of Celite, cooled to 60 C, then cooled to 10 C over 2 h. The resulting solids were collected by filtration, washing with 10 mL of 2-propanol. 3.35 g product was obtained (82% yield). |
|
In ethyl acetate; at 15 - 30℃; for 20h; |
50.1 mg of Tafamidis free acid and 31.7 mg of meglumine were mixed evenly, and were added into 2.5 mL of ethyl acetate. The mixture was stirred at room temperature for 20 hours to crystallize. White crystalline solid of Tafamidis meglumine was obtained by centrifugation and vacuum drying at room temperature. The obtained crystalline solid conformed to Form E of the present disclosure. Its XRPD pattern was substantially as depicted in FIG. 1, and the XRPD data were listed in Table 1. The DSC curve of Form E was substantially as depicted in FIG. 3, which comprises three endothermic peaks. Onset of the first endothermic peak is around 118 C., and the second is around 155 C. The third endothermic peak at 187 C. corresponds to the melting process. The thermal gravimetric analysis (TGA) curve of Form E was substantially as depicted in FIG. 4. It has approximate 2.6% weight loss when heated to 120 C. The 1HNMR spectrum was substantially as depicted in FIG. 5. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping