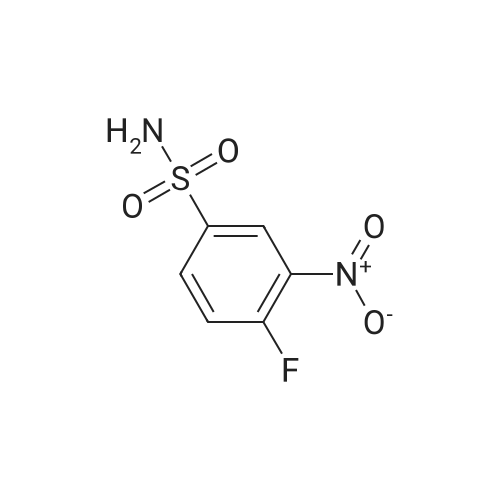
|
With palladium 10% on activated carbon; hydrogen; In methanol; dichloromethane; under 2585.81 Torr; for 0.25h; |
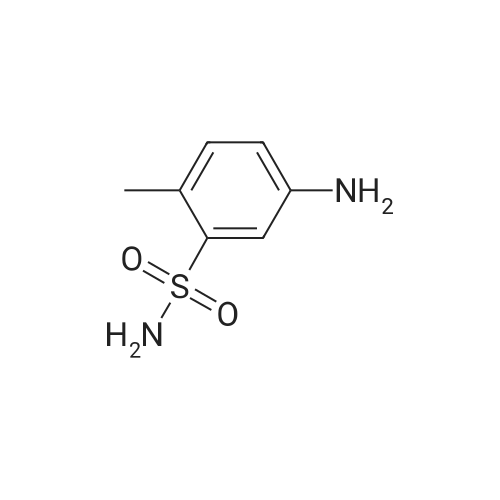
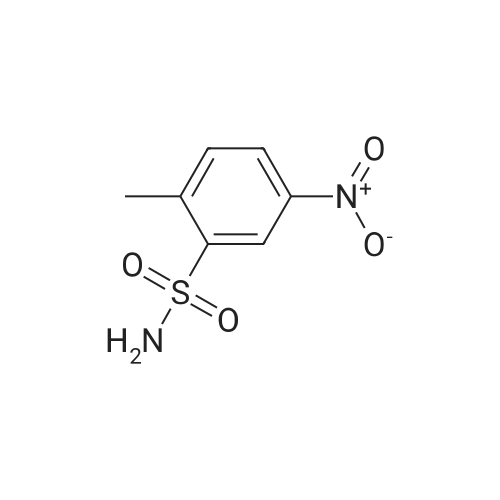
To a solution of 3-aminosulfonyl-4-methylnitrobenzene in dichloromethane and methanol was added 10percent Pd/C and the mixture shaken under a hydrogen atmosphere at 50 psi for 15 minutes. The mixture was filtered through diatomaceous earth and the filter cake was washed with methanol. The combined organic solvents were concentrated under reduced pressure to give crude product, which was further purified by flash column chromatography (ethyl acetate:hexanes 1:1) to give 3-aminosulfonyl-4-methylaniline, LCMS: purity: 87percent; MS (m/e): 187 (MH+). |
|
With palladium 10% on activated carbon; hydrogen; In methanol; dichloromethane; under 2585.81 Torr; for 0.25h; |
To a solution of 3-aminosulfonyl-4-methylnitrobenzene in dichloromethane and methanol was added 10percent Pd/C and the mixture shaken under a hydrogen atmosphere at 50 psi for 15 minutes. The mixture was filtered through diatomaceous earth and the filter cake was washed with methanol. The combined organic solvents were concentrated under reduced pressure to give crude product, which was further purified by flash column chromatography (ethyl acetate:hexanes 1:1) to give 3-aminosulfonyl-4-methylaniline, LCMS: purity: 87percent; MS (m/e): 187 (M+). |
|
With palladium 10% on activated carbon; hydrogen; In methanol; dichloromethane; under 2585.81 Torr; for 0.25h; |
To a solution of 3-aminosulfonyl-4-methylnitrobenzene in dichloromethane and methanol was added 10 percentPd/C and the mixture shaken under a hydrogen atmosphere at 50 psi for 15 minutes. The mixture was filtered throughdiatomaceous earth and the filter cake was washed with methanol. The combined organic solvents were concentratedunder reduced pressure to give crude product, which was further purified by flash column chromatography (ethyl acetate:hexanes 1:1) to give 3-aminosulfonyl-4-methylaniline, LCMS: purity: 87percent; MS (m/e): 187 (MH+). |
|
|
To a stirred solution of 2-methyl-5-nitrobenzenesulfonamide (4.6 g, 0.021 mol) in 2-methoxyethyl ether (43 mL), at 0° C., was added a solution of 16.1 g of tin(II) chloride in 32 mL of concentrated HCl dropwise over 15 min. After the addition was complete, the ice bath was removed and the solution was allowed to stir for an additional 30 min. Approximately 130 mL of diethyl ether was added to reaction. The mixture was stirred vigorously for 1 h. The mixture was basified with a solution of NaOH and NaHCO3, and extracted with ethyl acetate (*3). The combined ethyl acetate layers were dried over anhydrous MgSO4, filtered and concentrated to give crude product. Trituation of the crude product with methanol provided 2.4 g of pure 5-amino-2-methylbenzenesulfonamide as light brown solid. 1H NMR (300 MHz, DMSO-d6) delta 7.11-7.10 (m, 3H), 6.95 (d, J=8.1 Hz, 1H), 6.60 (dd, J=8.1 and 2.4 Hz, 1H), 5.24 (s, 2H), 2.36 (s, 3H). MS (ES+, m/z) 187 (M+H). |
|
With hydrogenchloride; tin(ll) chloride; In diethyl ether; diethylene glycol dimethyl ether; water; at 0℃; for 1.75h; |
Intermediate Example 5 Preparation of 5-amino-2-methylbenzenesulfonamide Procedure 1 To a stirred solution of 2-methyl-5-nitrobenzenesulfonamide (4.6 g, 0.021 mol) in 2-methoxyethyl ether (43 mL), at 0 °C, was added a solution of 16.1 g of tin(II) chloride in 32 mL of concentrated HCI dropwise over 15 min. After the addition was complete, the ice bath was removed and the solution was allowed to stir for an additional 30 min. Approximately 130 mL of diethyl ether was added to reaction. The mixture was stirred vigorously for 1 h. The mixture was basified with a solution of NaOH and NaHC03, and extracted with ethyl acetate (x 3). The combined ethyl acetate layers were dried over anhydrous MgS04, filtered and concentrated to give crude product. Trituation of the crude product with methanol provided 2.4 g of pure 5-amino-2- methylbenzenesulfonamide as light brown solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping