Alternatived Products of [ 624-49-7 ]
Product Details of [ 624-49-7 ]
| CAS No. : | 624-49-7 |
MDL No. : | MFCD00064438 |
| Formula : |
C6H8O4
|
Boiling Point : |
- |
| Linear Structure Formula : | HC(COOCH3)CHCOOCH3 |
InChI Key : | LDCRTTXIJACKKU-ONEGZZNKSA-N |
| M.W : |
144.13
|
Pubchem ID : | 637568 |
| Synonyms : |
NSC 25942;trans-Butenedioic Acid dimethyl ester;DMF;NSC 167432
|
Calculated chemistry of [ 624-49-7 ] Expand+
Physicochemical Properties
| Num. heavy atoms : |
10 |
| Num. arom. heavy atoms : |
0 |
| Fraction Csp3 : |
0.33 |
| Num. rotatable bonds : |
4 |
| Num. H-bond acceptors : |
4.0 |
| Num. H-bond donors : |
0.0 |
| Molar Refractivity : |
33.05 |
| TPSA : |
52.6 ?2 |
Pharmacokinetics
| GI absorption : |
High |
| BBB permeant : |
No |
| P-gp substrate : |
No |
| CYP1A2 inhibitor : |
No |
| CYP2C19 inhibitor : |
No |
| CYP2C9 inhibitor : |
No |
| CYP2D6 inhibitor : |
No |
| CYP3A4 inhibitor : |
No |
| Log Kp (skin permeation) : |
-7.02 cm/s |
Lipophilicity
| Log Po/w (iLOGP) : |
1.93 |
| Log Po/w (XLOGP3) : |
0.22 |
| Log Po/w (WLOGP) : |
-0.11 |
| Log Po/w (MLOGP) : |
0.15 |
| Log Po/w (SILICOS-IT) : |
0.2 |
| Consensus Log Po/w : |
0.48 |
Druglikeness
| Lipinski : |
0.0 |
| Ghose : |
None |
| Veber : |
0.0 |
| Egan : |
0.0 |
| Muegge : |
1.0 |
| Bioavailability Score : |
0.55 |
Water Solubility
| Log S (ESOL) : |
-0.61 |
| Solubility : |
35.5 mg/ml ; 0.247 mol/l |
| Class : |
Very soluble |
| Log S (Ali) : |
-0.88 |
| Solubility : |
18.8 mg/ml ; 0.131 mol/l |
| Class : |
Very soluble |
| Log S (SILICOS-IT) : |
-0.1 |
| Solubility : |
115.0 mg/ml ; 0.796 mol/l |
| Class : |
Soluble |
Medicinal Chemistry
| PAINS : |
0.0 alert |
| Brenk : |
2.0 alert |
| Leadlikeness : |
1.0 |
| Synthetic accessibility : |
2.12 |
Application In Synthesis of [ 624-49-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 624-49-7 ]
- 1
-
 [ 108030-46-2 ]
[ 108030-46-2 ]

-
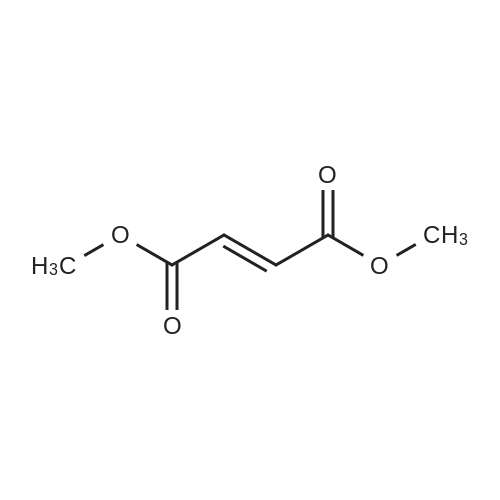 [ 624-49-7 ]
[ 624-49-7 ]

-
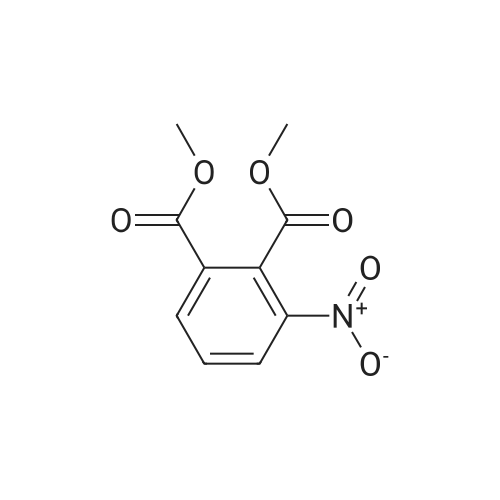 [ 13365-26-9 ]
[ 13365-26-9 ]

-
 [ 131-11-3 ]
[ 131-11-3 ]
- 2
-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 256658-45-4 ]
[ 256658-45-4 ]

-
 [ 78607-37-1 ]
[ 78607-37-1 ]

-
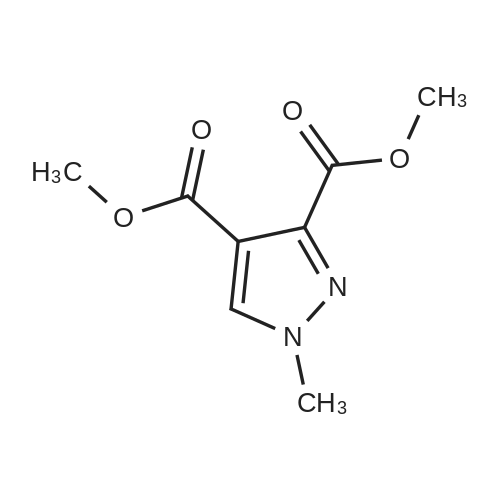 [ 22050-80-2 ]
[ 22050-80-2 ]

-
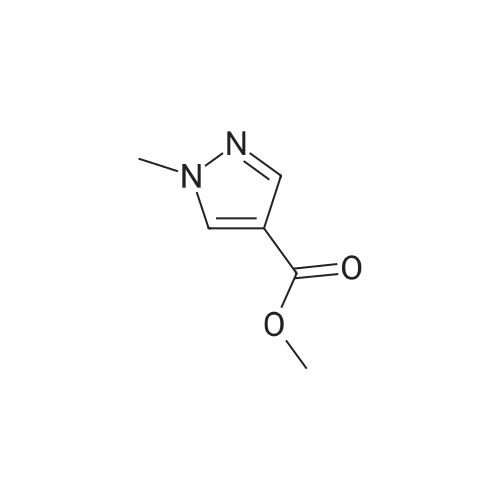 [ 5952-93-2 ]
[ 5952-93-2 ]
- 3
-
 [ 135616-40-9 ]
[ 135616-40-9 ]

-
 [ 147396-58-5 ]
[ 147396-58-5 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 137492-80-9 ]
[ 137492-80-9 ]
- 4
-
 [ 27567-82-4 ]
[ 27567-82-4 ]

-
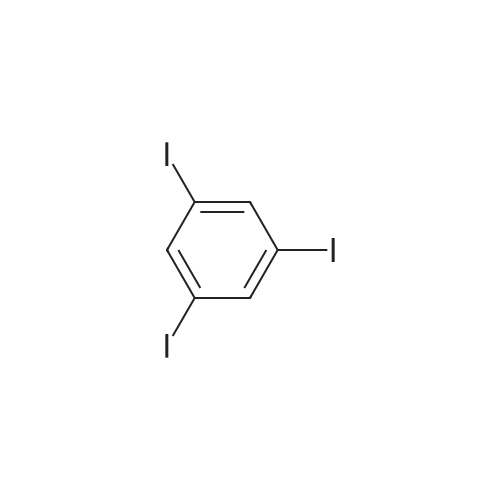 [ 626-44-8 ]
[ 626-44-8 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
1,3,5-tris(4',5'-dimethoxycarbonylspiro[2.5]oct-7'-ene-8'-yl)benzene
[ No CAS ]
- 5
-
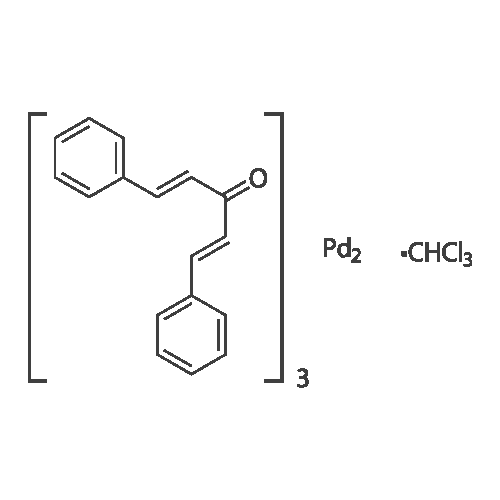 [ 52522-40-4 ]
[ 52522-40-4 ]

-
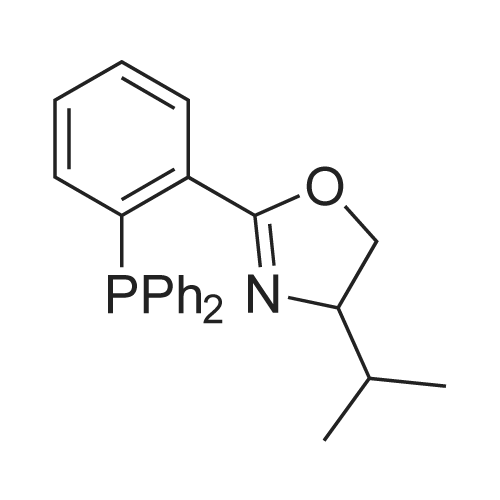 [ 594873-83-3 ]
[ 594873-83-3 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 500019-49-8 ]
[ 500019-49-8 ]
- 6
-
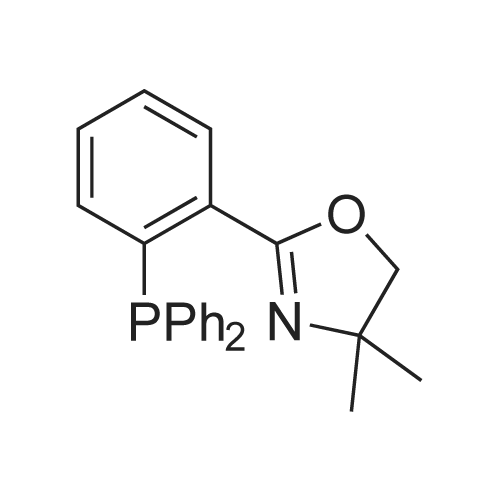 [ 64691-33-4 ]
[ 64691-33-4 ]

-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 499795-16-3 ]
[ 499795-16-3 ]
- 7
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 459489-07-7 ]
[ 459489-07-7 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 500019-41-0 ]
[ 500019-41-0 ]
- 8
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 189169-55-9 ]
[ 189169-55-9 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 500019-34-1 ]
[ 500019-34-1 ]
- 9
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 151644-44-9 ]
[ 151644-44-9 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 499795-20-9 ]
[ 499795-20-9 ]
- 10
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
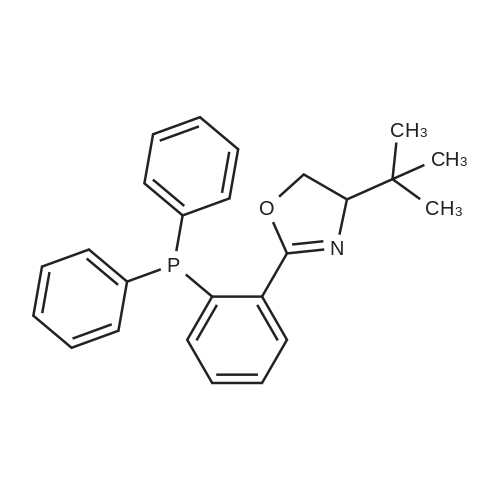
2-[2'-(diphenylphosphino)phenyl]-4,5-dihydro-4-methyl-4-phenyloxazole
[ No CAS ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 500019-28-3 ]
[ 500019-28-3 ]
- 11
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 444651-87-0 ]
[ 444651-87-0 ]

-
[1,2-bis[4,5-dihydro-3H-dibenzo[c-e]azepino]ethane](η2-dimethylfumarate)palladium
[ No CAS ]
- 12
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 99396-51-7 ]
[ 99396-51-7 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 1009811-15-7 ]
[ 1009811-15-7 ]
- 13
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 259796-48-0 ]
[ 259796-48-0 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
 [ 1009811-15-7 ]
[ 1009811-15-7 ]
- 14
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
C40H50NO4P
[ No CAS ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
C46H58NO8PPd
[ No CAS ]
- 15
-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
C40H50NO4P
[ No CAS ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
C46H58NO8PPd
[ No CAS ]
- 16
-
(S)-4-isopropyl-2-{2-[(3,3’,5,5’-tetra-tert-butyl-1,1’-biphenyl-2,2’-diyl)phosphite]-phenyl}-2-oxazoline
[ No CAS ]

-
 [ 52522-40-4 ]
[ 52522-40-4 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
C46H62NO8PPd
[ No CAS ]
- 17
-
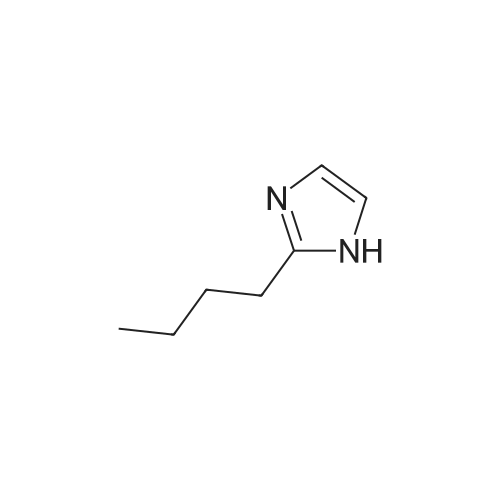 [ 50790-93-7 ]
[ 50790-93-7 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
dimethyl-2-(2-butylimidazol-1-yl)succinate
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 39% |
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetonitrile; at 25℃; for 3h; |
In a 100 ml flask with an inlet of four, 9.5 g (0.06 mol) of diazabicyclo undecene (DBU) , 18 ml of acetonitrile, 17.1 g (0.14 mol) of <strong>[50790-93-7]2-butylimidazole</strong> was added and the mixture was stirred at 25 C. In addition, 18.0 g (0.12 mol) of dimethyl fumarate was added dropwise and reacted at 25 DEG C for 3 hours. After completion of the reaction, the Extraction was carried out with 70 ml of methylene chloride and 50 ml of water, after removing the solvent under reduced pressure. The collected organic layer was concentrated by separation, and the obtained concentrate was purified by silica gel column chromatography (ethyl acetate / hexane = 1/1) , to obtain 2- (2-butyl-imidazol-1-yl) dimethyl succinate as a liquid phase. The obtained 2- (2-butyl-imidazol-1-yl) dimethyl succinate, yield 13.1g was 39%.2- (2-butylimidazol-1-yl) succinic acid dimethyl, and a release reaction of the protective group start under a temperature condition of 179 , and the disappearance of dimethyl succinate in 2- (2-butyl-imidazol-1-yl) dimethyl succinate was confirmed by NMR analysis. In addition, in GC analysis, it was confirmed that dimethyl fumarate originating from the leaving protecting group A was produced. |
- 18
-
 [ 623-73-4 ]
[ 623-73-4 ]

-
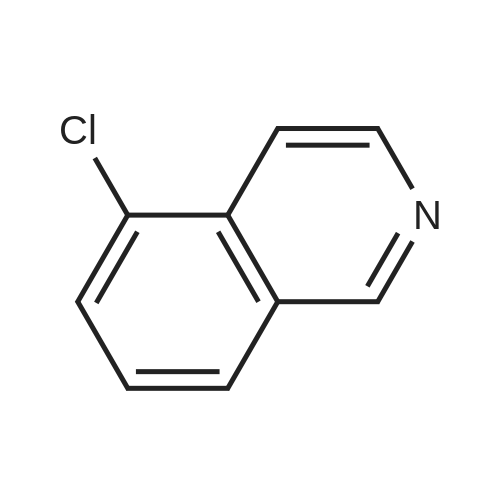 [ 5430-45-5 ]
[ 5430-45-5 ]

-
 [ 624-49-7 ]
[ 624-49-7 ]

-
3-ethyl 1,2-dimethyl 7-chloropyrrolo[2,1-a]isoquinoline-1,2,3-tricarboxylate
[ No CAS ]