|
With pyridine; at 200℃; for 0.5h;Inert atmosphere; Microwave irradiation; |
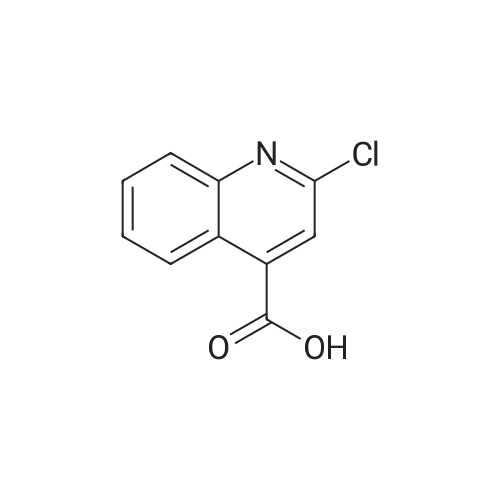
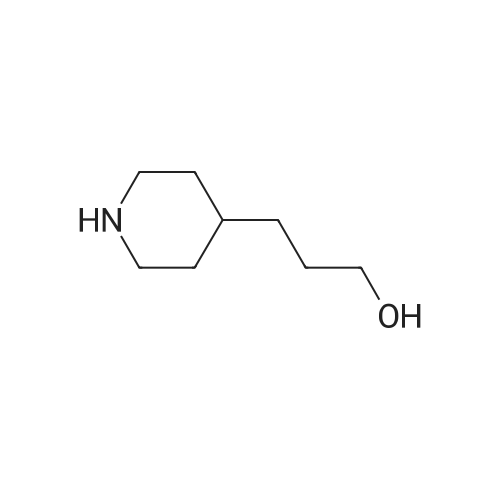
A mixture of <strong>[5467-57-2]2-chloroquinoline-4-carboxylic acid</strong> (0.95 g, 4.6 mmol) and 2-(piperidin-4-yl)ethanol (4.72 g, 36.5 mmol) in pyridine (10 mL) was heated to 200 °C for 30 min using a microwave reactor. Toluene was then added and the reaction mixture was concentrated in vacuo to give crude 2-[4-(2-hydroxyethyl)piperidin-1-yl]quinoline-4-carboxylic acid that was used with no further purification. A mixture of crude 2-[4-(2-hydroxyethyl)piperidin-1-yl]quinoline-4-carboxylic acid (4.56 mmol), sulfuric acid (0.97 mL, 18.2 mmol) in MeOH (20 mL) was heated at 120 °C for 30 min using a microwave reactor. Additional sulfuric acid (0.97 mL, 18.2 mmol) was added and the reaction mixture was heated 120 °C for 4 h using a microwave reactor. The reaction mixture was then partially evaporated and the residue partitioned between DCM and saturated aqueous NaHCO3. The aqueous phase was extracted with DCM (three times) and the combined organic phases were dried using a phase separator and concentrated in vacuo to leave a residue. The residue was purified by flash chromatography (50-->100percent EtOAc in heptane) to give methyl 2-[4-(2-hydroxyethyl)piperidin-1-yl]quinoline-4-carboxylate (1.03 g, 72percent). Oxalyl chloride (0.93 mL, 10.5 mmol) was added dropwise to a solution of DMSO (1.5 mL, 21.0 mmol) in DCM (45 mL) at -78 °C and the reaction mixture was stirred at -78 °C for 5 min. A solution of methyl 2-[4-(2-hydroxyethyl)piperidin-1-yl]quinoline-4-carboxylate (1.10 g, 3.51 mmol) in DCM (30 mL) was added and reaction mixture was stirred for 30 min at -78 °C. Triethylamine (6.8 mL, 49.1 mmol) was added and the reaction mixture was allowed to reach rt over 80 min. The reaction mixture was diluted with DCM and washed with H2O. The aqueous phase was extracted with DCM and the combined organic phases were dried (phase separator) and concentrated in vacuo to give the crude methyl 2-[4-(2-oxoethyl)piperidin-1-yl]quinoline-4-carboxylate, that was used with no further purification. Crude methyl 2-[4-(2-oxoethyl)piperidin-1-yl]quinoline-4-carboxylate (3.51 mmol) was dissolved in 2M dimethylamine (30 ml, 60 mmol) in MeOH. After 5 min sodium triacetoxyborohydride (3.72 g, 17.6 mmol) was added and the reaction mixture was stirred at rt for 2h. The reaction mixture was then concentrated in vacuo and the residue was partitioned between EtOAc and saturated aqueous NaHCO3. The aqueous phase was extracted with EtOAc (three times) and the combined organic phases were dried (Na2SO4) and concentrated in vacuo to leave a residue which was purified by flash column chromatography (0-->40percent MeOH in DCM) to give the title compound (0.92 g, 76 percent). 1H NMR (600 MHz, CDCl3) delta 8.41 - 8.37 (m, 1H), 7.71 (d, J = 8.4 Hz, 1H), 7.55 - 7.52 (m, 1H), 7.29 - 7.24 (m, 1H), 4.54 (d, J = 13.2 Hz, 2H), 4.00 (s, 3H), 3.02 - 2.91 (m, 2H), 2.40 - 2.32 (m, 2H), 2.24 (s, 6H), 1.82 (d, J = 12.4 Hz, 2H), 1.67 - 1.58 (m, 1H), 1.45 (dd, J = 15.0, 7.1 Hz, 2H), 1.33 - 1.24 (m, J = 12.6, 4.0 Hz, 2H); m/z (M+H)+ 342.2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping