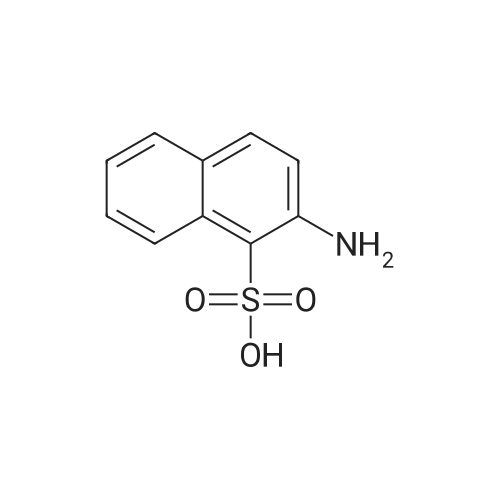
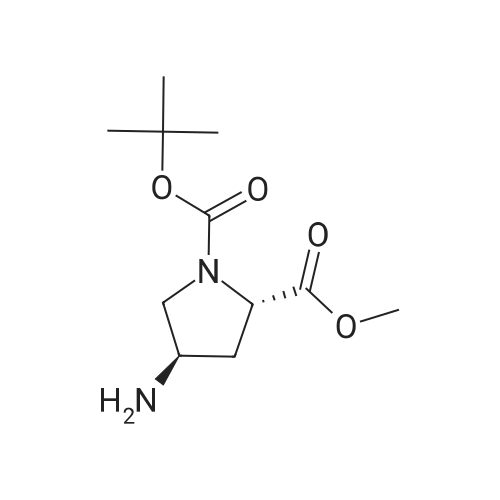
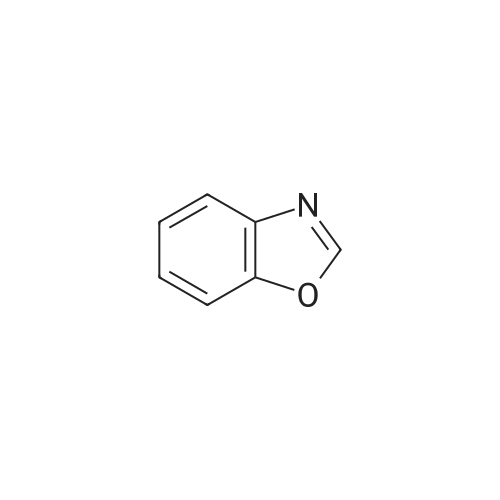
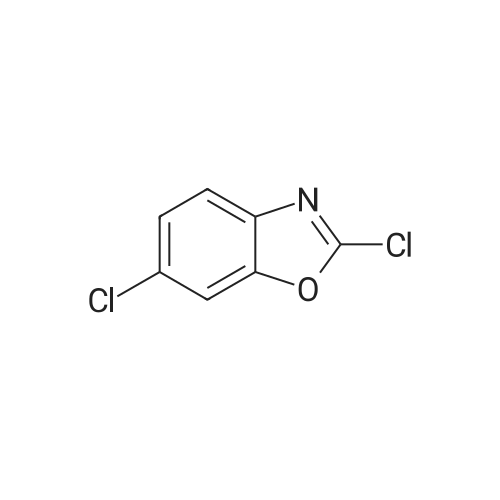
| 55% |
With triethylamine; In dichloromethane; at 0℃; for 0.5h; |
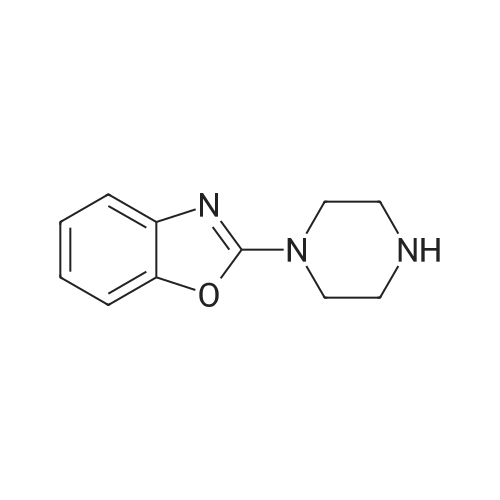
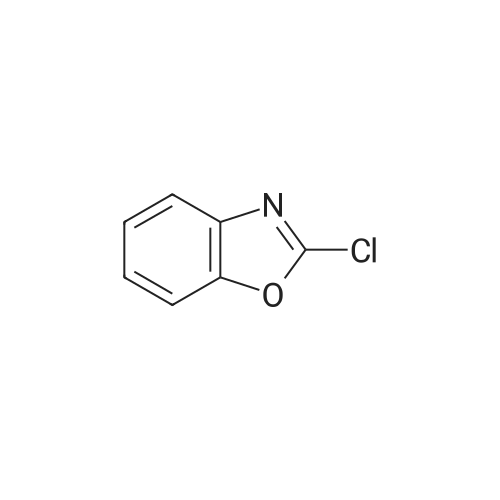
Step l 0.90 g (10.4 mmol) of piperazine was dissolved in 50 ml of dichloromethane in a dried round flask provided with nitrogen gas, 0.80 g (5.2 mmol) of 2-chlorobenzooxazole and 0.9 ml (52.1 mmol) of triethylamine were added thereto at 0C , and the mixture was incubated at 0C for 30 mins. After adding water thereto, the reaction mixture was extracted with ethyl acetate, and the formed organic layer was dried over anhydrous magnesium sulfate and concentrated under a reduced pressure. The resulting residue was subjected to silica gel column chromatography (dichloromethane:methanol=9:l) to obtain 0.58 g of 2-(piperazin-l-yl)benzooxazole (yield: 55%). |
|
With triethylamine; In dichloromethane; water; |
INVENTIVE EXAMPLE 14 2-(1-Piperazinyl)benzoxazole Anhydrous piperazine (5.6 g) was dissolved in methylene chloride (100 ml) to which was subsequently added triethylamine (4.5 ml). With cooling in an ice bath, to this was added dropwise 2-chlorobenzoxazole (3.7 ml) in small portions, followed by 45 minutes of stirring. The reaction solution was mixed with water, extracted with methylene chloride and then washed with saturated sodium bicarbonate aqueous solution and saturated brine in that order. The organic layer was dried with magnesium sulfate and the solvent was evaporated under a reduced pressure. Thereafter, the thus obtained mixture was purified by a silica gel column chromatography (methanol) to obtain the title compound 2-(1-piperazinyl)benzoxazole (4.751 g) in the form of yellow crystalline powder. |
|
With potassium carbonate;potassium iodide; In methanol; chloroform; acetonitrile; |
Reference example 16 Synthesis of 1-(2-benzoxazolyl)piperazine STR79 In 200 ml of acetonitrile were dissolved 33.6 g (0.39 mole) of piperazine and 10.0 g (0.065 mole) of 2-chlorobenzoxazole, and 9.0 g (0.065 mole) of potassium carbonate and a catalytic amount of potassium iodide were added thereto. The mixture was refluxed by heating for 11 hours under stirring. The mixture was cooled to room temperature and then filtered. The filtrate was concentrated under reduced pressure, and the residue was applied to silica gel column chromatography (the eluent used was a mixture of chloroform: methanol = 9: 1) to obtain 7.54 g of 1-(2-benzoxazolyl)piperazine as white crystals. MS spectrum (CI): m/e 204 (M+ +1) |
|
In dichloromethane; water; |
EXAMPLE 10 8-(Benzoxazol-2-yl)-8-aza-5-azoniaspiro[4,5]decane iodide A 3.07 g portion of 2-chlorobenzoxazole was dissolved in 10 ml of dichloromethane. Under cooling with ice, to this was added dropwise 3.45 g of anhydrous piperazine which has been dissolved in 30 ml of dichloromethane, followed by 1 hour of reaction. The reaction solution was concentrated under a reduced pressure, and the resulting residue was purified by a silica gel column chromatography (dichloromethane:methanol=20:1), followed by concentration of the elude under a reduced pressure. Thereafter, the resulting residue was dissolved in 30 ml of water, neutralized with 1N hydrochloric acid, extracted with dichloromethane, washed with water, dehydrated with Na2 SO4 and then concentrated under a reduced pressure to obtain 1.77 g of 2-(1-piperazinyl)benzoxazole. |
|
In ethanol; |
Preparation 4 1-(Benzoxazol-2-yl)piperazine STR22 To a stirred solution of piperazine (8 g, 93 mmole) in ethanol (30 ml) was added 2-chlorobenzoxazole (3 g, 19.5 mmole) dropwise. The resulting reaction was exothermic. The mixture was then stirred for 18 hours at room temperature, quenched by the addition of methylene chloride (50 ml) and the resulting precipitate removed by filtration. The filtrate was concentrated under reduced pressure then purified by flash column chromatography on silica gel eluding with methylene chloride: methanol: 0.88 ammonia solution (90:10:1) to yield the title compound, m.p. 70-72 (1.8 g, 46%), which was used directly. |
|
With potassium carbonate;potassium iodide; In methanol; chloroform; acetonitrile; |
(Reference example 16) Synthesis of 1-(2-benzoxazolyl)piperazine 33.6 g (0.39 mol) of piperazine and 10.0 g (0.065 mol) of 2-chlorobenzoxazole were dissolved in 200 ml of acetonitrile, and 9.0 g (0.065 mol) of potassium carbonate and a catalytic amount of potassium iodide were added thereto, followed by heating under reflux with stirring for 11 hours. The reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and the residue was subjected to silica gel column chromatography (eluding solution: mixture of chloroform:methanol = 9:1) to obtain 7.54 g of 1-(2-benzoxazolyl)piperazine as white crystals. MS spectrum (CI): m/e 204 (M++1) |
|
With triethylamine; In toluene; at 40℃; for 5h; |
To a solution of piperazine (2.24 g, 26 mmol, 1 equiv.) in toluene was added 2-chlorobenzoxazole (1.0 g, 6.51 mmol, 1 equiv.), followed by Et3N (3.62 mL, 4 equiv.). The resulting mixture was stirred at 40 C. for 5 hours. The solvent was removed under reduced pressure, and the residue was dissolved in EtOAc. The solution was washed with H2O (*4), brine and dried over Na2SO4. The solvent was evaporated under reduced pressure to afford 0.87 g of intermediate IV-A-17. |
|
In chloroform; at 20℃; for 16h; |
Step i) To a stirred solution of piperazine (560.9 mg, 6.50 mmol) in CHCl3 (16 ml_) was added 2-chlorobenzoxazole (500 mg, 3.25 mmol) at room temperature. The reaction mixture was stirred at room temperature for 16 hr. CHCl3 was removed and the resulting white solid was dissolved in water. After stirring in water for 30 min, the aqueous layer was extracted with CH2CI2. The combined organic layer was dried over MgSO4, filtered, and concentrated. The white solid (20, 439 mg, 67 %) was used for the next step without purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping