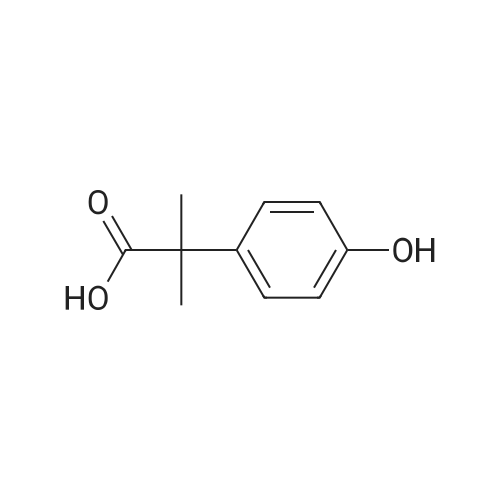
| 100% |
With borane-THF; In tetrahydrofuran; at -35 - 20℃; for 4h; |
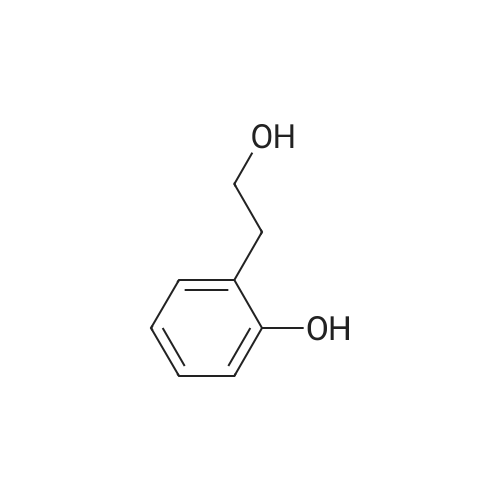
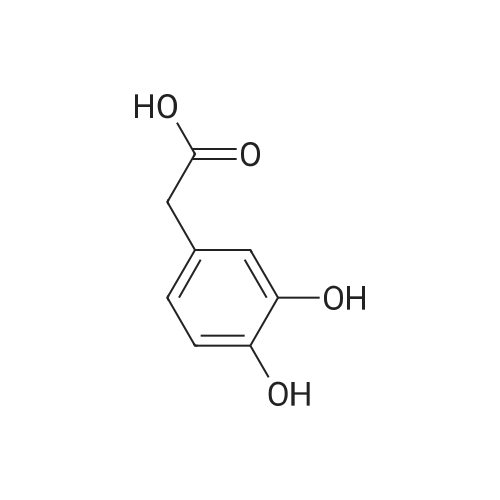
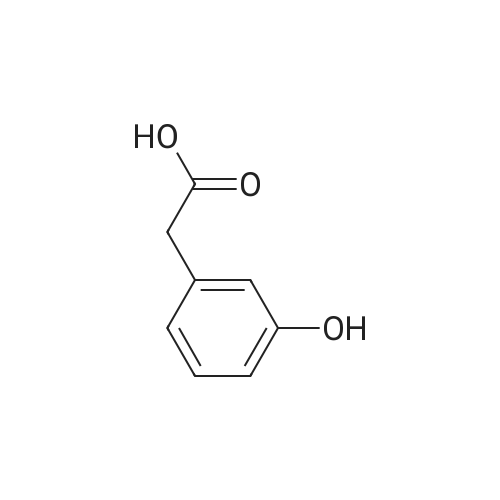
: 2-(2-hydroxyphenyl)acetic acid (5.0 g, 32 mmol) was dissolved in 160 mL of THF and cooled to -35°C. Borane (49 mL of 1.0 M in THF) was slowly added and the reaction warmed to ambient temperature. After 4 h, the mixture was cooled to 0°C and quenched with concentrated HC1. Once warmed to ambient, saturated ammonium chloride was added and THF removed by distillation under reduced pressure. The aqueous portion was extracted with ethyl acetate, which was then dried over MgS04, filtered, and concentrated to give the reduced alcohol in quantitative yield. NMR (400 MHz, CDC, delta): 2.19 (t, J = 8.0 Hz, 2 H), 4.01 (t, J = 8.0 Hz, 2 H), 6.92 (m, 4 H). |
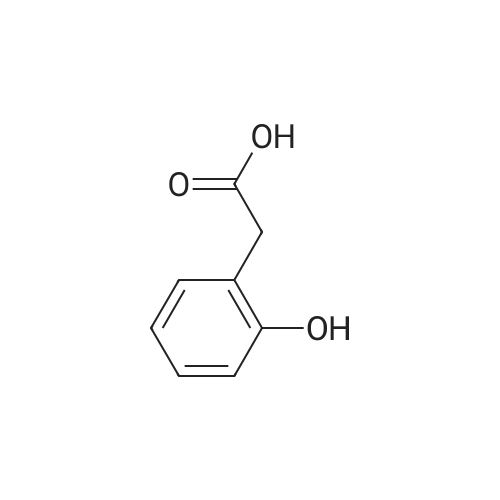
| 77% |
|
To a cold (0 °C) solution of 2-(2- hydroxyphenyl)acetic acid (10 g, 65.7 mmol) in THF (150 mL) was added Et3N (10.08 mL, 72.3 mmol) followed by ethyl chloroformate (6.31 mL, 65.7 mmol)dropwise. The mixture was stirred at 0 oc for 1 h and then solids were filtered andthe filterate was added to a cooled (0 °C) solution ofNaBH4 (3.73 g, 99 mmol) in50percent aqueous THF. The mixture was stirred at 0 oc for 1 hand then at room temp for 2 h. The solvent was removed in vacuo and the residue was digested in water (200 mL) and ether (500 mL). The ether layer was separated, washed with 2MNa2 C03, water, 1M citric acid and water, dried (Na2 S04), filtered and concentrated to afford 2-(2-hydroxyethyl)phenol (7 g, 50.7 mmol, 77percent yield) as colorless oil, which was used in the next step without purification. 1H NMR (500MHz, CDCh) 8 7.17(td, J=7.7, 1.7 Hz, IH), 7.09 (dd, J=7.5, 1.5 Hz, IH), 6.92 (dd, J=8.0, 1.0 Hz, IH),6.88 (td, J=7.4, 1.3 Hz, IH), 3.98 (dd, J=5.8, 5.0 Hz, 2H), 2.94- 2.88 (m, 2H). |
| 77% |
|
Intermediate 50 To a cold (0 °C) solution of 2-(2- hydroxyphenyl)acetic acid (10 g, 65.7 mmol) in THF (150 mL) was added Et3N (10.08 mL, 72.3 mmol) followed by ethyl chloroformate (6.31 mL, 65.7 mmol) dropwise. The mixture was stirred at 0 °C for 1 h and then solids were filtered and the filterate was added to a cooled (0 °C) solution of NaBH4 (3.73 g, 99 mmol) in 50percent aqueous THF. The mixture was stirred at 0 °C for 1 h and then at room temp for 2 h. The solvent was removed in vacuo and the residue was digested in water (200 mL) and ether (500 mL). The ether layer was separated, washed with 2M Na2C03, water, 1M citric acid and water, dried (Na2S04), filtered and concentrated to afford 2-(2-hydroxyethyl)phenol (7 g, 50.7 mmol, 77 percent yield) as colorless oil, which was used in the next step without purification. 1H NMR (500MHz, CDC13) 5 7.17 (td, J=7.7, 1.7 Hz, IH), 7.09 (dd, J=7.5, 1.5 Hz, IH), 6.92 (dd, J=8.0, 1.0 Hz, IH), 6.88 (td, J=7.4, 1.3 Hz, IH), 3.98 (dd, J=5.8, 5.0 Hz, 2H), 2.94 - 2.88 (m, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping