Synthesis and Exploration of Quinolino-Benzoxaboroles as Potential Therapeutic Agents
Kumpati, Greeshma P
;
University of Minnesota,2020.
More
Abstract: Benzoxaborole structure contains a phenyl ring fused with a heterocyclic oxaborole ring moiety. Benzoxaboroles are considerably more stable and exhibit high hydrolytic resistance compared with corresponding phenylboronic acids. The enhanced acidity of benzoxaboroles allows them to be predominantly in anionic forms in aqueous solution at physiological pH, which causes them to exhibit higher water solubility and better pharmacokinetic properties than phenylboronic acids. Increasing interest in benzoxaborole compounds is mainly due to their broad-spectrum biological activity including antimicrobial, anti-inflammatory and other medicinal properties. Quinoline is a highly privileged nitrogen containing a bicyclic ring system where a benzene ring is fused to a pyridine ring. The quinoline moiety is found in many natural products and has been traditionally used as a medicine for treating a wide variety of diseases. Quinoline-based molecules have been found to exhibit a diverse range of pharmacological properties with uses as antimalarial, antibacterial, anticonvulsant, cardiotonic, anticancer, anthelmintic, antifungal, anti-inflammatory and analgesic agents.
In this regard, we envisioned that introduction of aminobenzoxaborole unit on quinolines would result in novel molecular entities with favorable pharmacological and pharmaceutical properties for developing therapeutic agents for a wide variety of diseases. The aims of the current work include: 1) Develop a new synthetic methodology for the rapid creation of aminobenzoxaborole containing quinolines; and 2) Explore the efficacy of synthesized candidate compounds as antibacterial, antifungal, antiviral, antiinflammatory, and antimalarial agents.
As a part of this thesis, we developed a novel synthetic methodology for preparing quinolino aminobenzoxaboroles. The synthesized compounds were initially evaluated for their cytotoxic properties against various human and murine proliferating cancer cells. All the compounds were found to be well tolerated did not display toxicity even at high concentrations. Encouraged by their lack of toxicity, the test compounds were evaluated for their antibacterial activity against E. coli, B. subtilis, and M. smegmatis and for their antifungal activity against C. neoformans and C. albicans. Some of the synthesized derivatives exhibited good and selective activity against M. smegmatis. Future studies will involve evaluation of synthesized candidate compounds as antitubercular, antiviral, antimalarial, and anti-inflammatory agents.
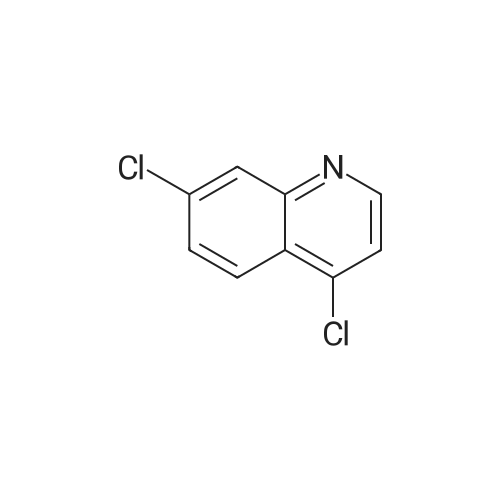
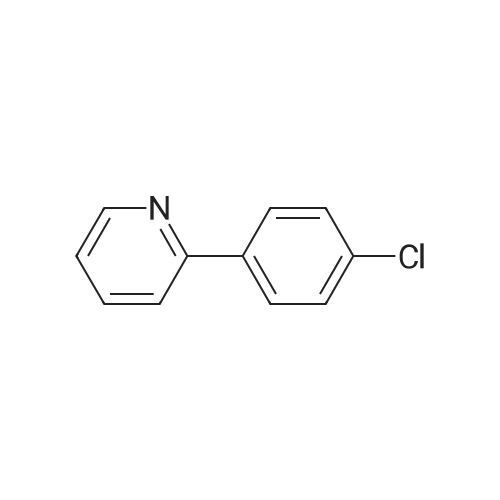
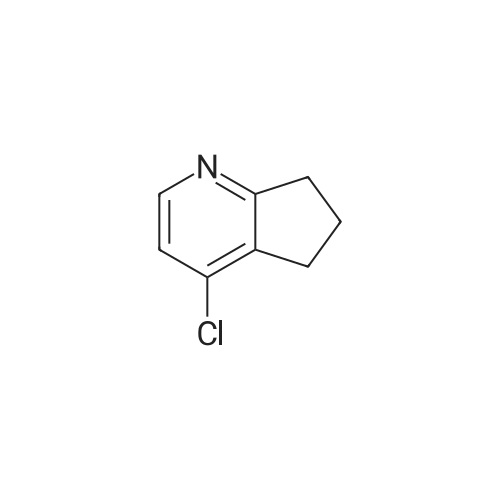
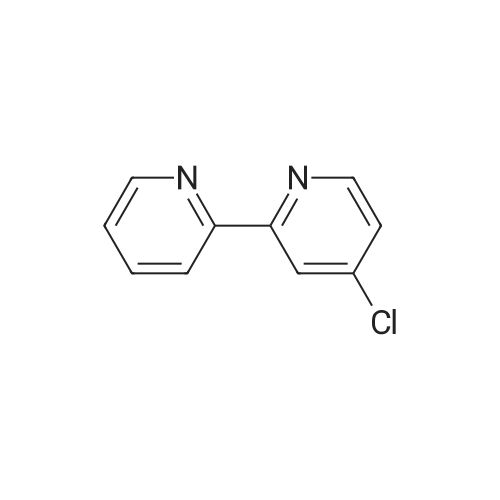
Purchased from AmBeed:
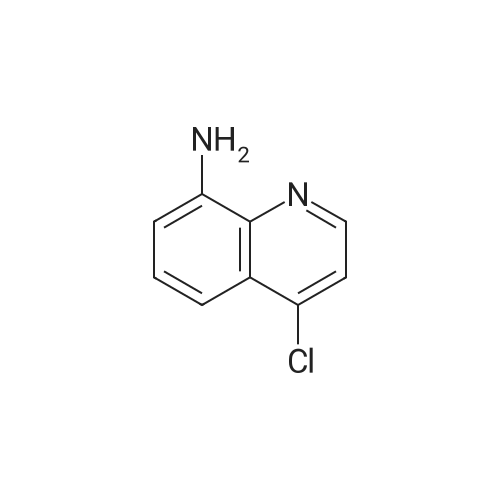
391-82-2 ;
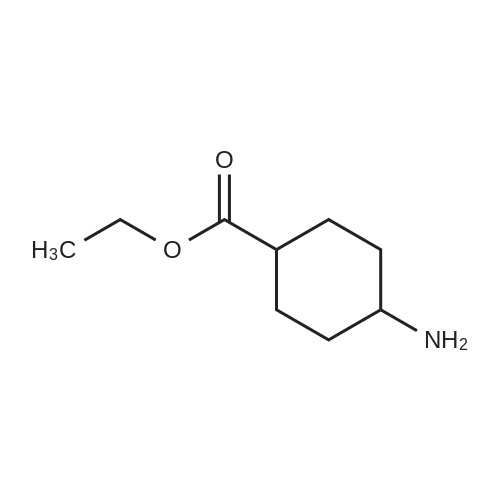
611-35-8 ;
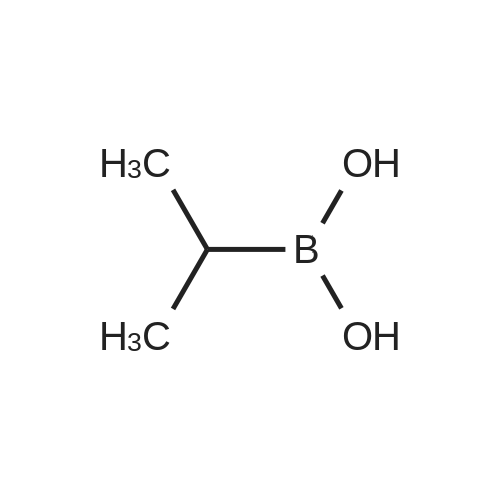
61964-08-7 ;
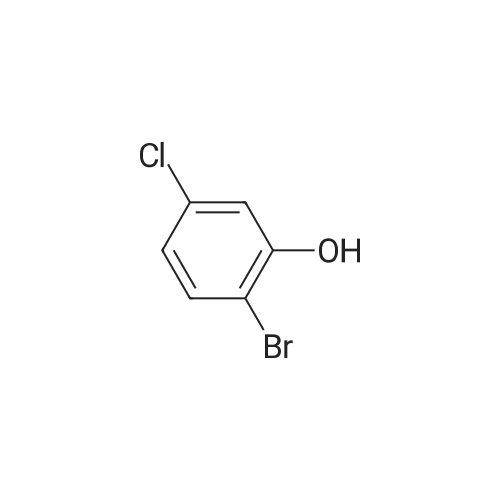
86-98-6 ;
284462-37-9 ;
89415-43-0

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping