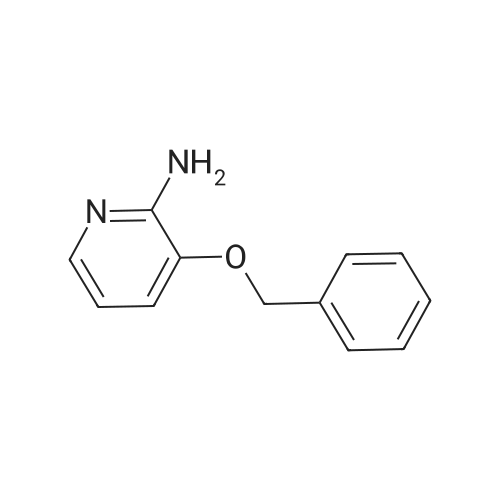
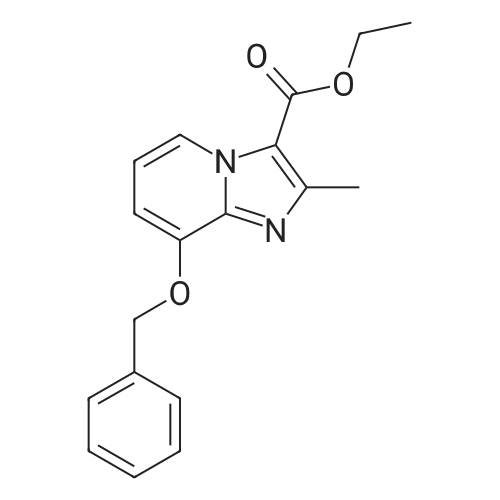
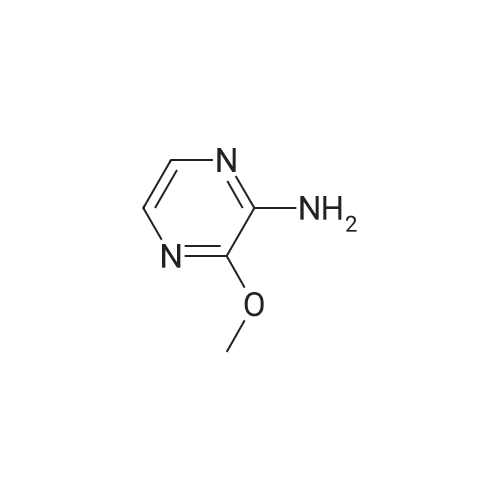
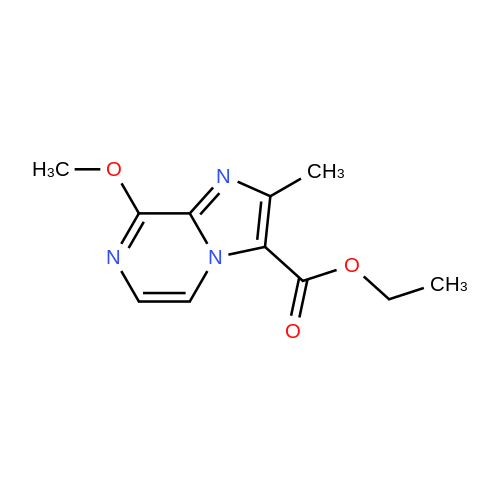
| 66% |
|
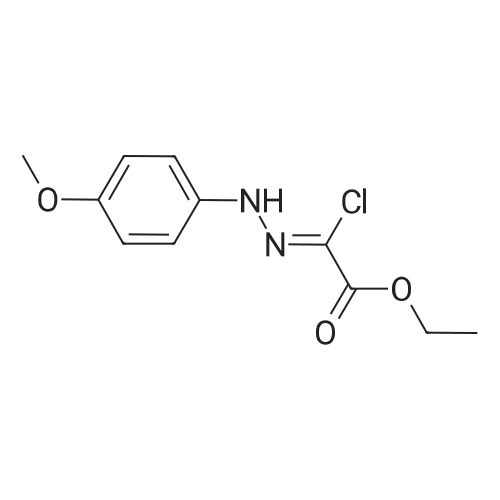
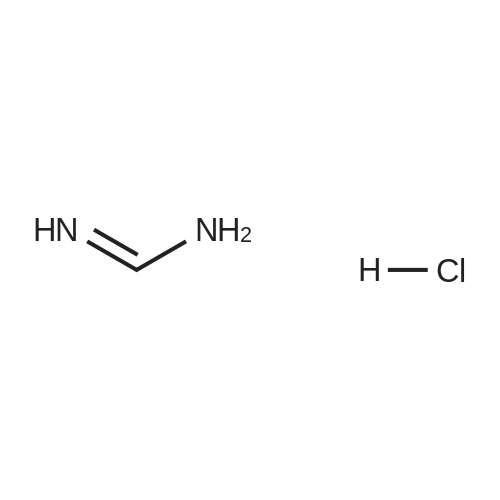
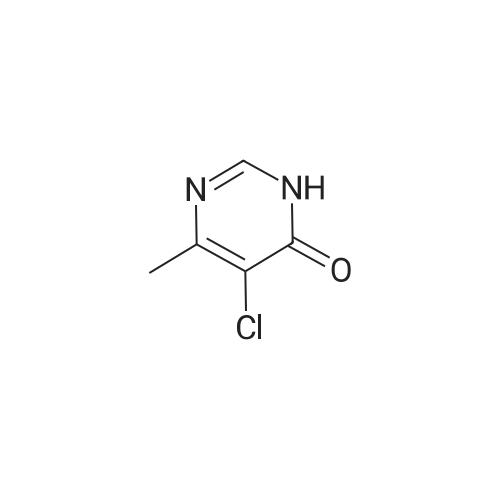
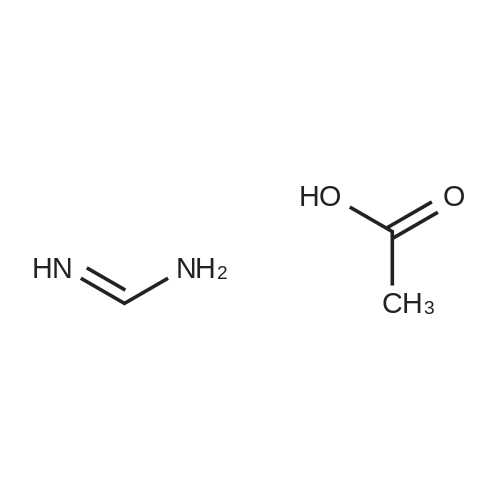
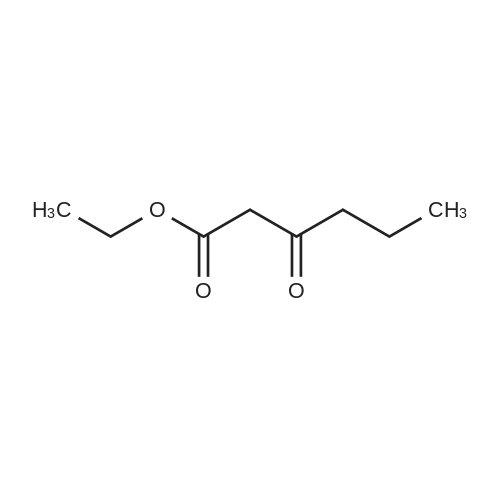
Stirring at room temperature to a solution of methanol 50ml 11.30g (0.11mol) of formamidine acetate was added slowly dropwise 8.80g (0.16mol) of sodium methoxide in methanol, stirring was continued at room temperature completion of dropwise 2h. Then added dropwise 11.17g (0.068mol) ethyl 2-chloroacetoacetate intermediate to the above solution, the reaction was stirred for 5-7 hours at room temperature continued. After completion of the reaction was monitored by TLC, the solvent was distilled off under reduced pressure, adjusted to pH = 5 with hydrochloric acid to 6, orange solid was suction filtered, the aqueous phase with (3 × 50ml) and extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered off solution. The residue was dissolved in 50ml of ethyl acetate and allowed to stand overnight, and filtered to give an orange solid 6.48g. Yield 66% |
| 66% |
|
To a solution of 11.30 g (0.11 mol) methylhydrazine acetate in 50 ml of methanol was slowly added dropwise 8.80 g with stirring at room temperature.(0.16 mol) of sodium methoxide in methanol, and the mixture was stirred at room temperature for 2 h. Then 11.17 g (0.068 mol) was added dropwise to the above solution.Ethyl 2-chloro-acetoacetate was stirred at room temperature for 5-7 hours. After TLC monitoring was completed, the solvent was distilled off under reduced pressure and salt was used.The pH was adjusted to 5-6, and the orange solid was filtered by suction. The aqueous phase was extracted with ethyl acetate (3×50 ml), dried over anhydrous magnesium sulfate, and dissolved.The combined product was dissolved in 50 ml of ethyl acetate, allowed to stand overnight and filtered to give 6.48 g of an orange-yellow solid. Yield 66.0% |
| 66% |
|
A solution of 8.80 g (0.16 mol) of sodium methoxide in methanol was slowly added dropwise to a solution of 11.30 g (0.11 mol) of methylacetoacetate in 50 ml of methanol at room temperature with stirring, and the mixturewas stirred at room temperature for 2 hours. Then, 11.17 g (0.068 mol) of the intermediate 2-chloroacetoacetic acid ethylacetate was added dropwise to theabove solution, and the reaction was continued for 5-7 hours while stirring at room temperature. After the TLCmonitoring reaction was completed, the solvent was evaporated under reduced pressure, and the pH wasadjusted to 5-6 with hydrochloric acid. The orange solid was collected by suction filtration, and the aqueousphase was extracted with ethyl acetate (3×50 ml), dried over anhydrous magnesium sulfate, filtered and shaken.Dissolved. The residue was dissolved in 50 ml of ethyl acetate, allowed to stand overnight and filtered to give 6.48g of an orange-yellow solid. The yield of 66%. |
| 66% |
|
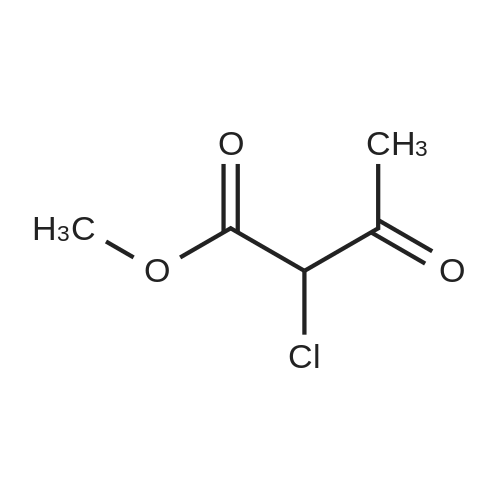
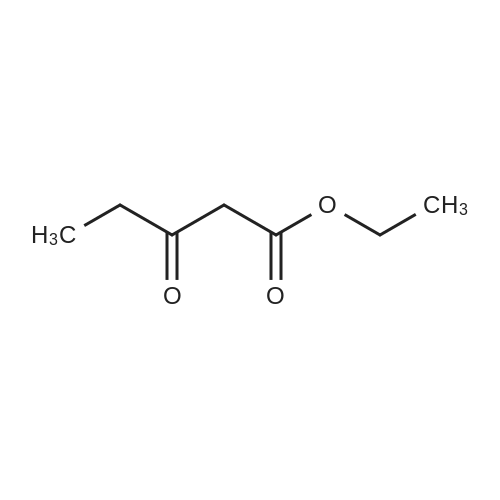
10343] A solution of 8.80 g (0.16 mol) of CH3ONa in methanol was added slowly to a solution of 11.30 g (0.11 mol) of formimidamideacetate in 50 mL of methanol at room temperature under stirring, the mixture was stirred for another 2 h after addition at room temperature. Followed by addition of 11.17 g (0.068 mol) of ethyl 2-chloro-3-oxobu- tanoate, the mixture was continued stirring for another 5-7 h at room temperature. Afier the reaction was over by Thin-Layer Chromatography monitoring, the reaction mixture was concentrated under reduced pressure and pH was adjusted to 5-6 with HC1, and then filtered to afford orangeyellow solid, the water phase was extracted with ethyl acetate (3x50 mL), dried over anhydrous magnesium sulfate, filtered and then concentrated under reduced pressure. The residue was dissolved to 50 ml of ethyl acetate, stand overnight to obtain 6.48 g as orange-yellow solid with yield of 66%. m.p. 181-184 C. |
| 66% |
|
To a solution of 11.30 g (0.11 mol) of formamidine acetate in 50 ml of methanol, a solution of 8.80 g (0.16 mol) of sodium methoxide in methanol was slowly added dropwise with stirring at room temperature.Stirring was continued for 2 h at room temperature.Then, 11.17 g (0.068 mol) of an intermediate ethyl 2-chloroacetoacetate was added dropwise to the above solution, and the reaction was further stirred at room temperature for 5-7 hours.After the TLC monitoring reaction was completed, the solvent was evaporated under reduced pressure, and the mixture was adjusted to pH 5 to 6 with hydrochloric acid, and filtered to give an orange-yellow solid.The aqueous phase was extracted with (3 × 50ml) of ethyl acetate, dried over anhydrous magnesium sulfate, filtered, desolventized.The residue was dissolved in 50ml of ethyl acetate, allowed to stand overnight, and filtered to give an orange-yellow solid 6.48g. Yield 66%, |
| 66% |
|
To a solution of 11.30 g (0.11 mol) formamidine acetate in 50 ml of methanol under stirring at room temperature, 8.80 g (0.16 mol) of sodium methoxide in methanol was slowly added dropwise, and stirring was continued at room temperature for 2 h.Then, 11.17 g (0.068 mol) of intermediate ethyl 2-chloroacetoacetate was added dropwise to the above solution, and the reaction was continued to be stirred at room temperature for 5-7 hours.After the reaction was monitored by TLC, the solvent was distilled off under reduced pressure, and the pH was adjusted to 5-6 with hydrochloric acid.Filtration with suction yielded an orange-yellow solid, and the aqueous phase was extracted with (3 × 50 ml) ethyl acetate, dried over anhydrous magnesium sulfate, filtered and desolvated.The residue was dissolved in 50 ml of ethyl acetate, left overnight, and filtered to obtain 6.48 g of orange solid. The yield is 66%, |
| 66% |
|
To a solution of 11.30 g (0.11 mol) formamidine acetate in 50 ml of methanol under stirring at room temperature, 8.80 g (0.16 mol) of sodium methoxide in methanol was slowly added dropwise, and stirring was continued at room temperature for 2 h. Then, 11.17g (0.068mol) of intermediate ethyl 2-chloroacetoacetate was added dropwise to the above solution, and the reaction was stirred at room temperature for 5-7 hours. After the reaction was monitored by TLC, the solvent was distilled off under reduced pressure, and the pH was adjusted to 5 with hydrochloric acid. 6, suction filtration to obtain orange-yellow solid, the aqueous phase was extracted with (3 × 50ml) ethyl acetate, dried over anhydrous magnesium sulfate, filtered and desolvated. The residue was dissolved in 50 ml of ethyl acetate, left overnight, and filtered to obtain 6.48 g of orange solid. 66% yield |
|
With sodium methylate; In methanol; at 5 - 20℃; |
General procedure: A solution of formamidine acetate (0.70 mol) in methanol (150 mL)was stirred at 5-10 C, and then CH3ONa (1.20 mol) in methanol(150 mL) newly prepared was added slowly under stirring, followed byaddition of M-1 (0.50 mol) in methanol (100 mL). The mixture wascontinued stirring for another 3-4 h at room temperature and thenconcentrated under reduced pressure. The residue was adjusted to pH5-6 with diluted HCl, the precipitated solid was filtered to afford M-2 asa white solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +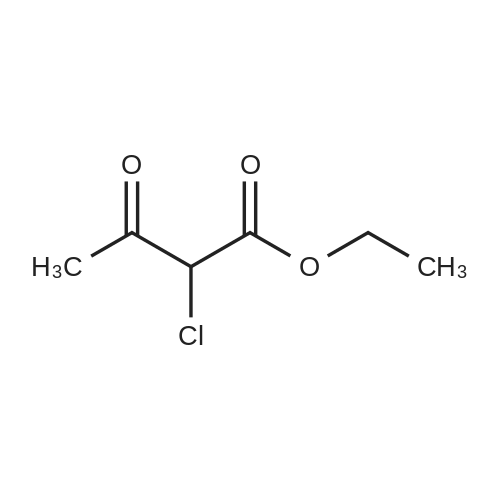

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping