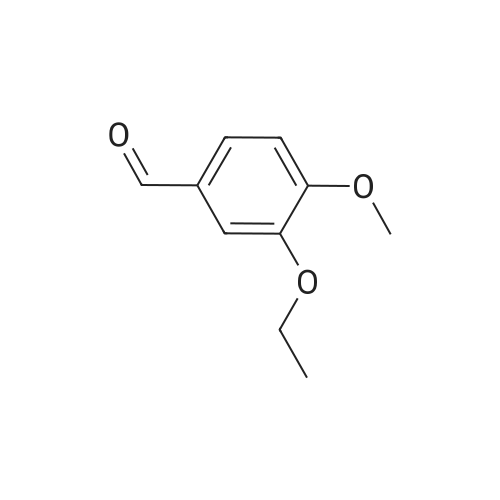
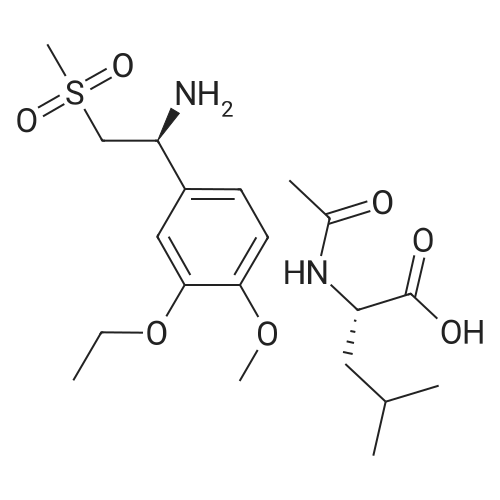
| 73.5% |
at 60 - 70℃; for 2h; |
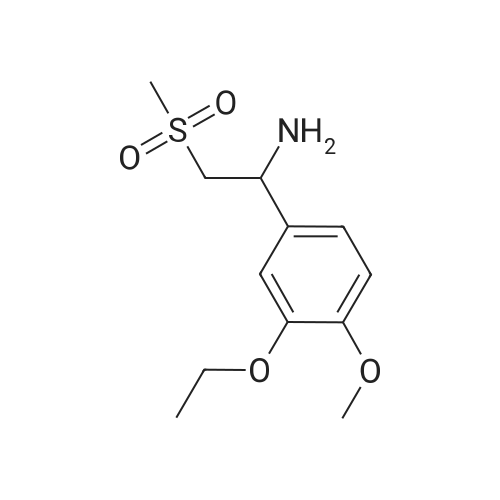
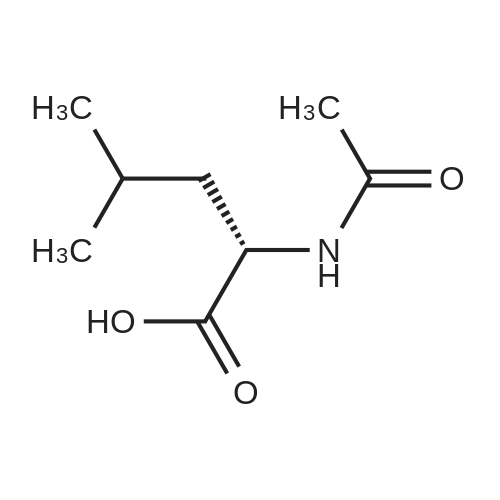
In a 500 ml round bottom flask, 200 ml of methanol was added followed by 20 gms of 2-(3-ethoxy-4-methoxyphenyl)-l-(methanesulfonyl)-eth-2-ylamine. The reaction mass was stirred and 76 gms of N-Acetyl-L-Leucine added and reaction mass was stirred. The reaction mass was heated for 2 hours at 60 to 65C, After heating, the reaction masswas cooled at room temperature and it was stirred at room temperature for 3 to 4 hours. The slurry was filtered. Washed with 30 ml methanol, material was unloaded and dried under vacuum for 2 hours at 45C. Yield: 14.36 gm. Further, this material is purified with methanol. |
| 41% |
In methanol; for 2h;Reflux; |
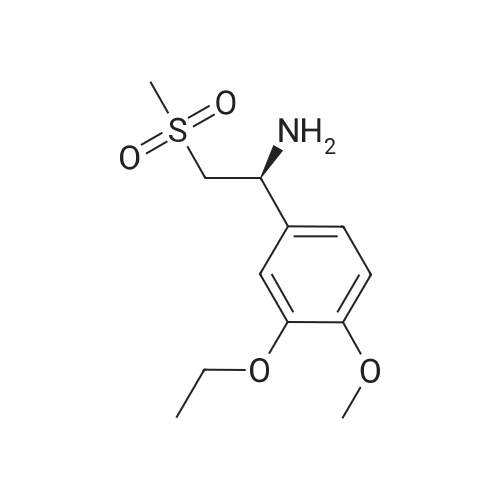
A mixture of 27.3 g (100 mmol) of 1- (3-ethoxy-4-methoxy) phenyl-2-methanesulfonyl ethylamine (4), 10.4 g(60 mmol) of N-acetyl-L-leucine was dissolved in 200 mL of methanol, heated under reflux for 2 h, and then cooled to room temperature to a large amountThe slurry was precipitated, filtered and washed with methanol to give the crude product. The crude product was again dissolved in 80 mL of methanol, heated to reflux for 2 h, and cooled againTo a large amount of slurry to the slurry, filter, and wash the filter cake with methanol. The solid was dried in vacuo to give 9.1 g (41% recovery)(S) -1- (3-ethoxy-4-methoxy) phenyl-2-methanesulfonylethylamine N-acetyl-L-leucine salt (97.9% ee). |
| 35% |
In methanol; for 1h;Inert atmosphere; Reflux; |
Example 5: Preparation of (S)-1-(4-methoxy-3-ethoxyphenyl)-2-methanesulfonylethylamine N-acetyl-L-leucine salt (IV) A dry 1,000mL glass flask equipped with a mechanical stirrer, a thermometer, and a condenser was added 500g of methanol, 136 g (0.5mol) <strong>[253168-94-4](R,S)-1-(4-methoxy-3-ethoxyphenyl)-2-methanesulfonylethylamine</strong> (E.g. racemate III prepared in Example 1), and 86.5 g (0.5mol) N-acetyl-L-leucine under nitrogen protection. The solution was heated to reflux for 1 hour. The solution was then cooled to 10C and filtered. The resultant filtrate will be used as mother liquor in Example 6. The resulting filter cake was washed with 55g of cold methanol 10C and dried under vacuum to give 78.1 g of white solid (S)-1-(4-methoxy-3-ethoxyphenyl)-2-methanesulfonylethylamine N-acetyl-L-leucine salt (IV). Yield 35.0%, optical purity 99.8%. |
|
In methanol; at 20℃; for 8h;Heating / reflux; |
A mixture of <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L) was charged into a 3-L 3-necked round bottom flask equipped with a mechanical stirrer, a thermometer, and a condenser. After the reaction mixture was refluxed for 1 hour, the mixture was allowed to cool to ambient temperature and then stirred for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuum at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuum at 30 C. to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). Chiral HPLC (1/99 EtOH/20 mM KH2PO4 (at)pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies, 150 mm×4.6 mm, 0.5 mL/min., (at)240 nm): 18.4 min (S-isomer, 99.2%), 25.5 min (R-isomer, 0.8%). |
|
In methanol; |
6.2.3. Resolution of <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C. to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). Chiral HPLC (1/99 EtOH/20 mM KH2PO4pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies, 150 mm*4.6 mm, 0.5 mL/min., 240 nm): 18.4 min (S-isomer, 99.2%), 25.5 min (R-isomer, 0.8%). |
|
In methanol; at 20℃; for 4h;Reflux; |
[0113j A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with 2-(3-ethoxy-4-methoxyphenyl)-1 - (methylsulphonyl)-eth-2-ylamine (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C to a constant weight, yielding 49.6 g (90% recovery) of (S)-2-(3 -ethoxy-4-methoxyphenyl)- 1 -(methylsulphonyl)-eth-2-ylamine -Nacetyl-L-leucine salt (98.4% ee). Chiral HPLC (1/99 EtOH/20 mM KH2PO4 pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies, 150mm x 4.6 mm, 0.5 mL/min., 240 nm): 18.4 mm (S-isomer, 99.2%), 25.5 mm (R-isomer, 0.8%). |
| 49.6 g |
In methanol; for 1h;Reflux; |
6.2.3. Resolution of <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C. to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). |
|
In methanol; for 1h;Reflux; |
Resolution of 2-(3-ethoxy-4-methoxyphenyl-1-(methylsulphonyl)-eth-2-ylamine A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C. to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). |
| 18 g |
In methanol; at 25℃; for 4h;Reflux; |
The compound of formula V 0. 1mol 200mL of methanol was added and the reaction flask was refluxed clear solution was added portionwise N- acetyl-L- leucine (0.06mol ), a large number of the resulting solid was refluxed 1h, cooled to 25 C, stirred 1h, filtered and the filter cake was dried in vacuo at 40 C 5h, to give a white solid 22. 6g; The solid was added to the reaction flask and 180mL of methanol, refluxed for 1h, cooled to 25 C, stirred for 1h, filtered and the filter cake in and dried in vacuo 40 C 5h, to give a white solid 18g, 40 C and dried in vacuo 5h, ee: 98 · 6% |
| 68 g |
In methanol; at 25℃; for 2h;Reflux; |
To a reaction mixture of 700 ml methanol and 100 grams (gm) (0.366 moles) of 1-(3-ethoxy-4- methoxy phenyl)-2-(methylsulfonyl) ethanamine, 38gm (0.2196 moles) of N-acetyl-L-leucine was charged. The reaction mixture was heated to reflux for 1 hour. The reaction mixture was cooled to 25C to 30C and stirred for 1 hour. The product was filtered and washed with 100 ml methanol.The obtained solid material was dried under vacuum at 30C to obtain (S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl) ethanamine N-acetyl-L-leucine salt.(Yield = 68.Ogms)R-isomer: 0.5% |
| 35 g |
In methanol; at 60 - 65℃; for 1h; |
To 1 liter RBF the product of example 3 or 4 (75 g; 0.2743 moles), N- acetyl-L-leucine (47.5 g ; 0.2743) and methanol (545 mL) were charged and the suspension was stirred at 60 C to 65 C for 1 hour, the suspension was cooled to room temperature and stirred for another 3 hours. The solid was filtered and dried under reduced pressure to obtain 60 g of title compound with chiral purity of 85% desired isomer. It was further purified by methanol to give 35 g of pure title compound having chiral purity 99.5%. |
|
In methanol; at 60 - 65℃; for 1h; |
To 1 liter round bottom flask, 1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethanamine,N-acetyl-L-leucine and methanol were charged and the suspension was stirred at 60 C to 65C for 1 hour, the suspension was cooled to room temperature and stirred for another 3 hours.The solid was filtered and dried under reduced pressure to obtain the title compound withchiral purity of 85 % desired isomer. Yield: 40 %.It was further purified by methanol to give pure title compound having chiral purity 99.5% |
| 49.6 g |
In methanol; for 1h;Reflux; |
A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). Chiral HPLC (1/99 EtOH/20 mM KH2PO4 pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies, 150 mm x 4.6 mm, 0.5 mL/min., 240 nm): 18.4 min (S-isomer, 99.2%), 25.5 min (R-isomer, 0.8%). |
| 49.6 g |
In methanol; for 1h;Reflux; |
A 3 L 3-necked round bottom flask was equipped with a mechanical stirrer, thermometer, and condenser and charged with <strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong> (137.0 g, 500 mmol), N-acetyl-L-leucine (52 g, 300 mmol), and methanol (1.0 L). The stirred slurry was heated to reflux for 1 hour. The stirred mixture was allowed to cool to ambient temperature and stirring was continued for another 3 hours at ambient temperature. The slurry was filtered and washed with methanol (250 L). The solid was air-dried and then dried in vacuo at ambient temperature to a constant weight, giving 109.5 g (98% yield) of the crude product (85.8% ee). The crude solid (55.0 g) and methanol (440 mL) were brought to reflux for 1 hour, cooled to room temperature and stirred for an additional 3 hours at ambient temperature. The slurry was filtered and the filter cake was washed with methanol (200 mL). The solid was air-dried and then dried in vacuo at 30 C to a constant weight, yielding 49.6 g (90% recovery) of (S)-<strong>[253168-94-4]2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulphonyl)-eth-2-ylamine</strong>-N-acetyl-L-leucine salt (98.4% ee). Chiral HPLC (1/99 EtOH/20 mM KH2PO4 pH 7.0, Ultron Chiral ES-OVS from Agilent Technologies, 150 mm x 4.6 mm, 0.5 mL/min., 240 nm): 18.4 min (S-isomer, 99.2%), 25.5 min (R-isomer, 0.8%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping