Synergistic photoinactivation of Escherichia coli and Listeria innocua by curcumin and lauric arginate ethyl ester micelles
Ryu, Victor
;
Chuesiang, Piyanan
;
Corradini, Maria G.
, et al.
LWT,2023,173,114317.
DOI:
10.1016/j.lwt.2022.114317
More
Abstract: This study evaluated the changes in dispersibility in the aqueous phase, chem. stability, and antimicrobial activity of curcumin after being encapsulated in a lauric arginate Et ester (LAE) micelle. Stock curcumin-LAE solutions were prepared by titrating curcumin dissolved in ethanol into LAE aqueous solutions (pH 3.5). The LAE in the stock solutions inhibited the crystallization and prevented the chem. degradation of curcumin during storage at 20°C. The antimicrobial activity of the curcumin-LAE solutions against Escherichia coli (E. coli) and Listeria innocua (L. innocua) cocktails was assessed by exposing the sample to UV-A light (λ = 365 nm) for 5 min. For samples with both LAE and curcumin at pH 3.5 during irradiation, synergistic antimicrobial activity was observed The release of protein or nucleic acid from the cells indicated an increase in its membrane permeability after treatments which was due to LAE-facilitated interaction between the photosensitizer and the membrane. LAE could inactivate both bacteria within 10 min without UV-A light irradiation, only at pH 7, which shows that LAE's antimicrobial efficacy depends on the pH. Therefore, microbial inactivation by two mechanisms, photosensitization and permeability operating simultaneously, produced a curcumin-LAE solution that could inactivate bacteria at a broad pH range.
Keywords:
Microbial photoinactivation ;
Curcumin ;
Lauric arginate ethyl ester ;
Micelles ;
Photosensitizer
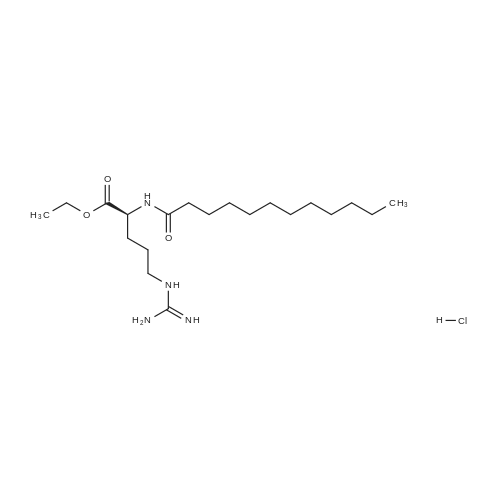
Purchased from AmBeed:
60372-77-2

Mechanism of Synergistic Photoinactivation Utilizing Curcumin and Lauric Arginate Ethyl Ester against Escherichia coli and Listeria innocua
Ryu, Victor
;
Uknalis, Joseph
;
Corradini, Maria G.
, et al.
Foods,2023,12(23):4195.
DOI:
10.3390/foods12234195
PubMed ID:
38231609
More
Abstract: This study investigated the mechanism of how lauric arginate Et ester (LAE) improves the photoinactivation of bacteria by curcumin after diluting the 100 μmol/L stock curcumin-LAE micelle solution to the concentration used during the treatment based on the curcumin concentration The photoinactivation of bacteria was conducted by irradiating the 1 μmol/L curcumin-LAE solution containing cocktails of Escherichia coli and Listeria innocua strains (7 log CFU/mL) for 5 min with UV-A light (λ = 365 nm). The changes in solution turbidity, curcumin stability, and bacterial morphol., viability, and recovery were observed using SEM, TEM, and live/dead cell assays. The study found that LAE enhances the photoinactivation of bacteria by increasing the permeability of cell membranes which could promote the interaction of reactive oxygen species produced by photosensitized curcumin with the cell components. The combination of curcumin and LAE was demonstrated to be more effective in inhibiting bacterial recovery at pH 3.5 for E. coli, while LAE alone was more effective at pH 7.0 for L. innocua.
Keywords:
microbial photoinactivation ;
curcumin ;
lauric arginate ethyl ester ;
photosensitizer ;
reactive oxygen species
Purchased from AmBeed:
60372-77-2

Creating new water‐soluble Iso‐ and n‐fatty acid arginate hydrochloride with antimicrobial properties
Jianwei Zhang
;
Kun Huang
;
Xuetong Fan
, et al.
Eur. J. Lipid Sci. Tech.,2023,125(11):2300012.
DOI:
10.1002/ejlt.202300012
More
Abstract: Typically, short- and long-chain lipids from oils exhibit different antimicrobial activities and therefore have been used in agriculture and aquaculture, biomedical therapeutic and antibacterial fields. However, these fatty acids have limitations in terms of bioactive efficacy, thermostability and aqueous solubility. In this study, water-soluble iso-fatty acid arginate hydrochloride derivatives with antimicrobial properties were produced by introducing branched (iso-) chain and other linear- (n-) chain fatty acids to the "arginine" amino acid molecule. The two-step synthetic route was straightforward and provided an efficient 88% and 76% product yields for ethyl n-oleoyl arginate hydrochloride and ethyl iso-oleoyl arginate hydrochloride, respectively. ATR-FT-IR, NMR, and LC-MS-Q-TOF techniques were used to thoroughly characterize and confirm the products. These arginate products had strong antimicrobial activities against Listeria innucua, a Gram-positive bacterium with minimum inhibitory concentrations and minimum bactericidal concentrations ranging from 1.8 μg mL[?1] to 29.1 μg mL[?1]. Therefore, the study demonstrated the development of a novel class of antimicrobial compounds from iso-fatty acids and arginates.
Keywords:
antimicrobial ;
ethyl lauroyl arginate ;
iso-fatty acid arginate ;
iso-oleic acid ;
isostearic acid
Purchased from AmBeed:
60372-77-2


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping