| 34% |
|
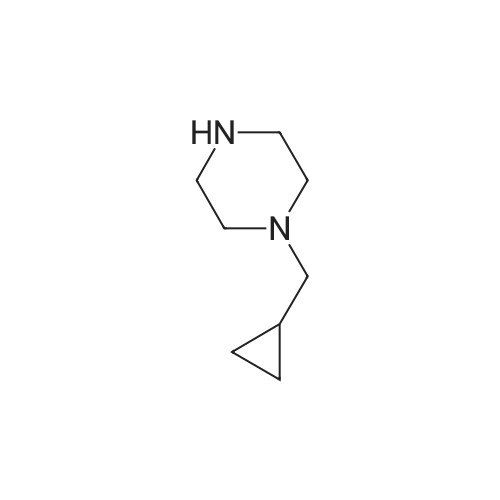
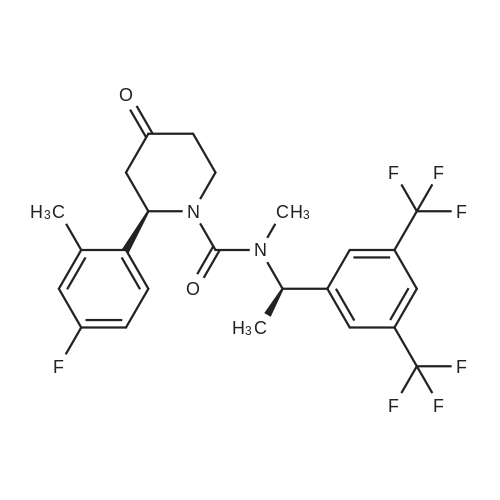
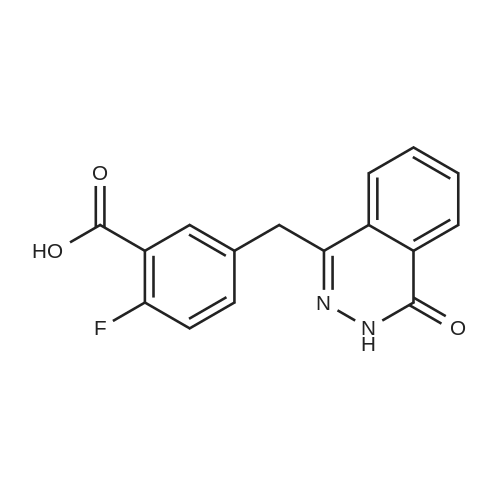
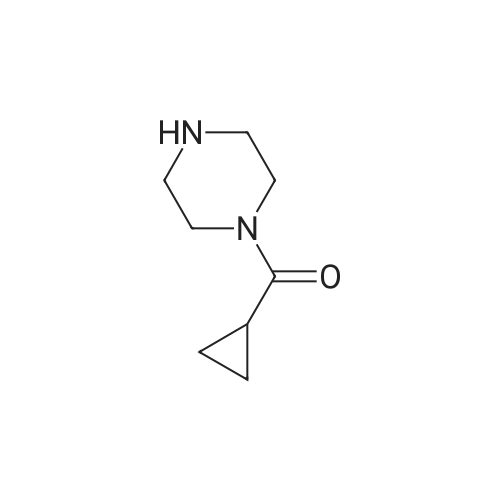
To a solution of 2-Fluoro-5-((4-oxo3,4-dihydrophthalazin-l-yl)methyl)benzoic acid (50 mg, 0.168 mmol) in DMA (1 mL) was added DIPEA (56 L, 0.336 mmol) and HBTU (64 mg, 0.170 mmol). The reaction mixture was stirred for 1 hour before addition of cyclopropylpiperazine-l-ylmethanone (0.170 mmol) was carried out. The reaction mixture was stirred at room temperature for 48 h. The reaction mixture was then extracted with DCM (3 x 5 mL) and washed with water (3 x 20 mL). The organic layers were collected, dried with MgS04 and the excess solvent removed in vacuo. Purification via reverse phase HLPC was carried out affording 4-(3-(4 (cyclopropanecarbonyl) piperazine- l-carbonyl)-4- fluorobenzy phthalazin- 1 (2//)-one (olaparib) (25 mg, 34%) as a white solid. *H NMR (400 MHz, CDCh) d = 10.65 (s, 1H), 8.44 - 8.37 (m, 1H), 7.75 - 7.61 (m, 3H), 7.34 - 7.22 (m, 2H), 6.97 (t, J = 8.9 Hz, 1H), 4.22 (s, 2H), 3.90 - 3.09 (m, 8H), 1.79 - 1.52 (s, 3H), 0.99 - 0.88 (m, 2H), 0.81 - 0.63 (s, 2H); {}1^ NMR (376 MHz, CDCb) d = - 117.6; Mp: 69 - 7lC. Data is in accordance with known literature (Menear, K.A., et al., ibid.). |
|
With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 3 - 20℃; for 3.5h;Product distribution / selectivity; |
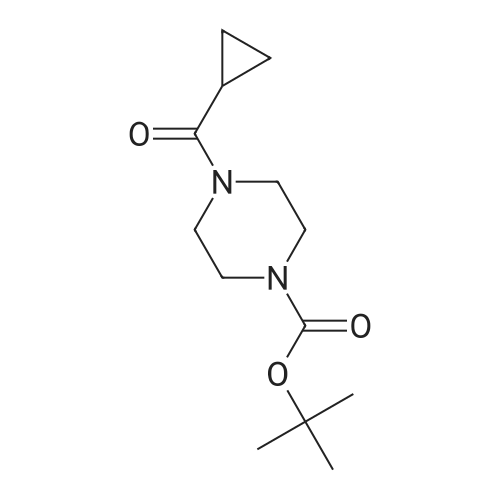
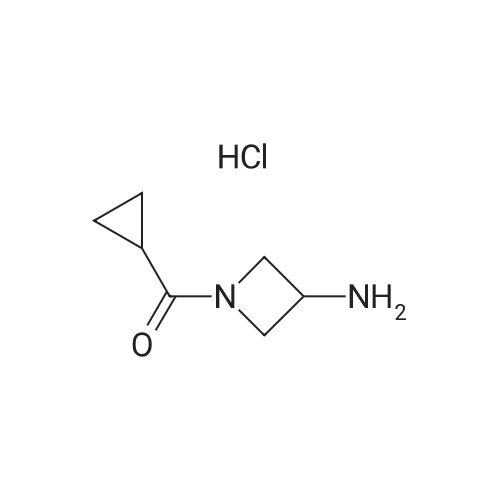
Example 2: Alternative synthesis of Compound A using i-fcyclopropylcarbonyl) piperazineMethods (also for Examples 3 & 4)NMR1H NMR spectra were recorded using Bruker DPX 400 spectrometer at 400 MHz. Chemical shifts were reported in parts per million (ppm) on the delta scale relative to tetramethylsilane internal standard. Unless stated otherwise all samples were dissolved in DMSOd6.Mass SpectraMass spectra were recorded on an Agilent XCT ion trap mass spectrometer using tandem mass spectrometry (MS/MS) for structural confirmation. The instrument was operated in a positive ion elctrospray mode.(a) 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one (Compound A)2-Fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid (D)(15.23g, 51.07 mmol) was suspended with stirring under nitrogen in acetonitrile (96 ml). Diisopropylethylamine (19.6 ml, 112.3 mmol) was added followed by 1-cyclopropylcarbonylpiperazine (l)(9.45g, 61.28 mmol) and acetonitrile (1 ml). The reaction mixture was cooled to 18C. O-Benzotriazol-1-yl- tetramethyluronium hexafluorophosphate (25.18g, 66.39 mmol) was added over 30 minutes and the reaction mixture was stirred for 2 hours at room temperature. The reaction mixture was cooled to 3C and maintained at this temperature for 1 hour, before being filtered. The filter cake was washed with cold (3C) acetonitrile (20 ml) before being dried in vacuo at up to 4O0C to give the title compound as a pale yellow solid (20.21 g).Mass Spectrum: MH+ 4351H NMR (400MHz. DMSO-d6) delta: 0.70 (m, 4H), 1.88 (br s, 1 H), 3.20 (br s, 2H), 3.56 (m, 6H), 4.31 (s, 2H), 7.17 (t, 1 H), 7.34 (dd, 1 H), 7.41 (m, 1 H), 7.77 (dt, 1 H), 7.83 (dt, 1 H), 7.92 (d, 1 H), 8.25 (dd, 1 H), 12.53 (s, 1 H). |
|
With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 3 - 20℃; for 3.5h; |
(a) 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one (Compound A)2-Fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid (D)(15.23g, 51.07 mmol) was suspended with stirring under nitrogen in acetonitrile (96 ml). Diisopropylethylamine (19.6 ml, 112.3 mmol) was added followed by 1-cyclopropylcarbonylpiperazine (l)(9.45g, 61.28 mmol) and acetonitrile (1ml). The reaction mixture was cooled to 18C. O-Benzotriazol-1-yl- tetramethyluronium hexafluorophosphate (25.18g, 66.39 mmol) was added over 30 minutes and the reaction mixture was stirred for 2 hours at room temperature. The reaction mixture was cooled to 3C and maintained at this temperature for 1 hour, before being filtered. The filter cake was washed with cold (3C) acetonitrile (20 ml) before being dried in vacuo at up to 400C to give the title compound as a pale yellow solid (20.21 g).Mass Spectrum: MH+ 4351H NMR (400MHz, DMSO-d6) delta: 0.70 (m, 4H), 1.88 (br s, 1H), 3.20 (br s, 2H), 3.56 (m, 6H), 4.31 (s, 2H), 7.17 (t, 1H), 7.34 (dd, 1 H), 7.41 (m, 1H), 7.77 (dt, 1H), 7.83 (dt, 1H), 7.92 (d, 1H), 8.25 (dd, 1 H)1 12.53 (S1 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping