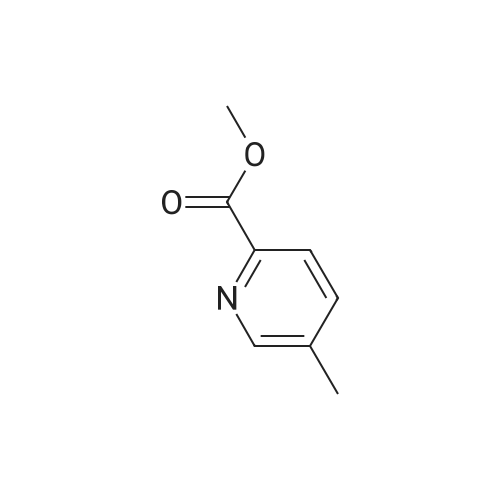
| 89.9% |
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 0 - 20℃; for 16.25h; |
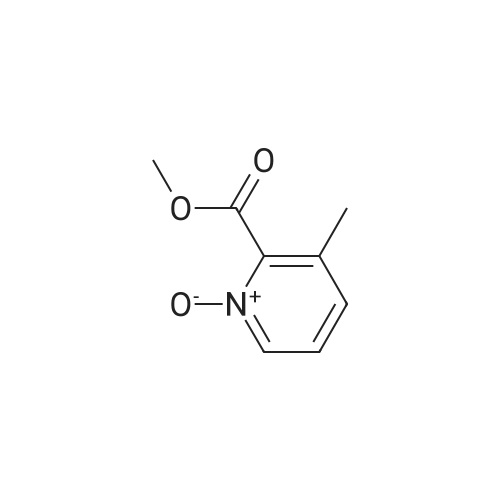
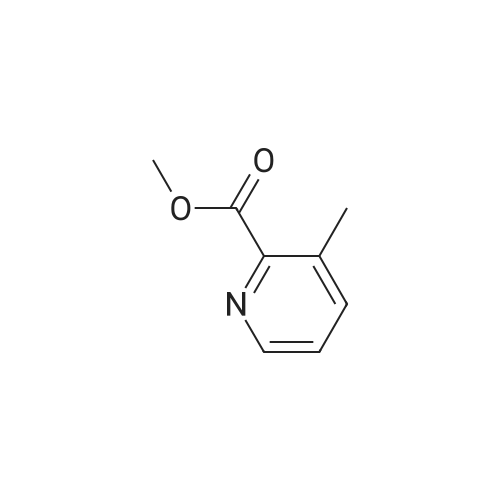
To a solution of <strong>[59718-84-2]methyl 3-methylpyridine-2-carboxylate</strong> (13.0 g, 0.086 mol) in CH2CI2 (130 mL) is added meta-chloroperoxybenzoic acid (89.05 g, 0.258 mol, 50 % w/w) portionwise at 0 C. The reaction mixture is stirred for 15 minutes at 0C and then gradually warmed to ambient temperature. After 16 hours, saturated NaHC03 solution (100 mL) is added. The mixture is stirred for 30 minutes and is extracted with CH2CI2. The combined organic layers are washed with 0.5M NaOH aqueous solution (2 χ 50 mL), dried over sodium sulfate, filtered, and concentrated under reduced pressure to give methyl 3 -methyl- 1 -oxido-pyridin- 1 -ium-2-carboxylate as an off- white solid (13.0 g, 89.9%). The residue is used in the next step without further purification. Mass spectrum (m z): 168.2 (M+H)+. |
| 85% |
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20℃; for 24h;Inert atmosphere; |
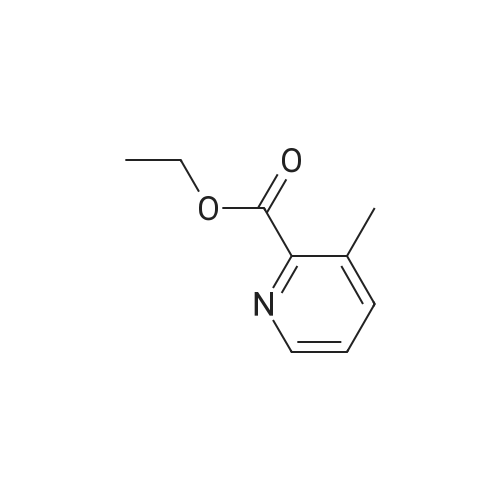
To a solution of ethyl 2-methyl-3-pyridinecarboxylate (1.86 ml_, 12.1 mmol) in DCM (50 ml_) mCPBA was added (4.18 g, 24.2 mmol) at RT. The solution was stirred at RT for 24 hrs. The solution was filtered and concentrated. The residue was purified by FC on silica (eluent: DCM to 15% MeOH) to a solid still containing mCBA that was dissolved with DCM and washed with NaHC03, the organic phase was dried and evaporated to afford 2- (methoxycarbonyl)-3-methylpyridin-N oxide (p134, 1.9 g, y=85%). MS (m/z): 168.1 [M]+. |
| 79% |
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 20℃; |
A mixture of <strong>[59718-84-2]methyl 3-methylpicolinate</strong> (2 g, 14.8 mmol) and m-CPBA (3 g, 17.8 mmol) in DCM (35 mL) was stirred at RT overnight. Then the mixture was washed with NaHSC (a.q.) and concentrated in vacuum. The residue was purified by column chromatography (DCM : MeOH = 200 : 1) to give the product of 2-(methoxycarbonyl)-3- methylpyridine 1-oxide (1.89 g, yield: 79%). -NMR (CDC13> 400 MHz) δ 8.05 (d, J= 6.0 Hz, 1H), 7.18 (t, J= 8.4 Hz, 1H), 7.10 (d, J= 8.0 Hz, 1H), 3.95 (s, 3H), 2.23 (s, 3H). MS (M+H)+: 168. |
| 79% |
With 3-chloro-benzenecarboperoxoic acid; In dichloromethane; at 25℃; |
A mixture of <strong>[59718-84-2]methyl 3-methylpicolinate</strong> (2 g, 14.8 mmol) and m-CPBA (3 g, 17.8mmol) in DCM (35 mE) was stirred at RT overnight. Then the mixture was washed with NaHSO3 (a.q.) and concentrated in vacuum. The residue was purified by column chromatography (DCM:MeOH = 200: 1) to give the product of 2-(methoxycarbonyl)-3-methylpyridine 1-oxide (1.89 g, yield: 79%). ‘H-NMR (CDC13, 400 MHz) 8.05 (d, J 6.0 Hz, 1H), 7.18 (t, J 8.4 Hz, 1H), 7.10 (d, J= 8.0 Hz, 1H), 3.95 (s, 3H), 2.23 (s, 3H). MS (M+H): 168. |
|
With dihydrogen peroxide; In water; acetic acid; at 20 - 60℃; |
Example 716-[4-Chloro-3-[[(tricyclo[3.3.1.13'7]dec-l-ylmethyl)amino]carbonyl]phenyl]-3-methyl-2-pyridinecarboxylic acidCla) 3-Methyl-2-pyridinecarboxyIic acid 1-oxide, methyl esterA solution of 3-methyl 2-pyridinecarboxylic acid, methyl ester (340 mg) in acetic acid (5mL) and 35% aqueous hydrogen peroxide (5 mL) was heated at 60C for 5 hours beforebeing stirred at room temperature overnight. The reaction mixture was quenched bypouring into a sodium sulfite/ ice water mixture (50 mL) and then extracted intodichloromethane (3 x 25 mL). The organics were dried over magnesium sulphate, filteredand concentrated to dryness to give the sub-title compound as a colourless oil (300 mg).MS: APCI(+ve) 168 (M+H4). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping