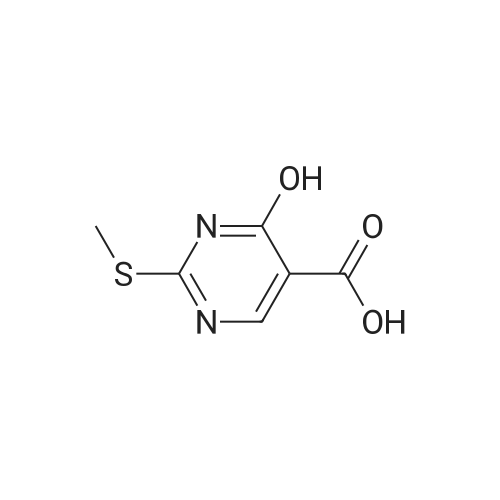
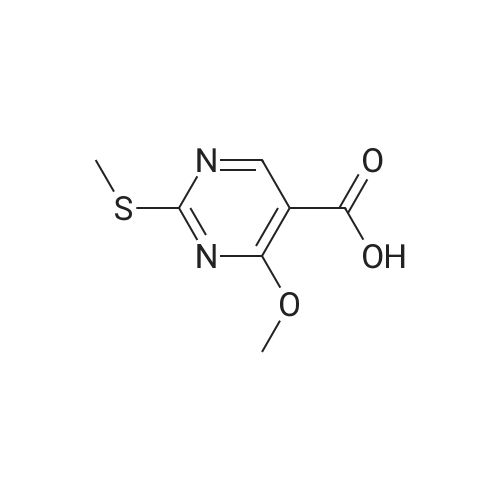
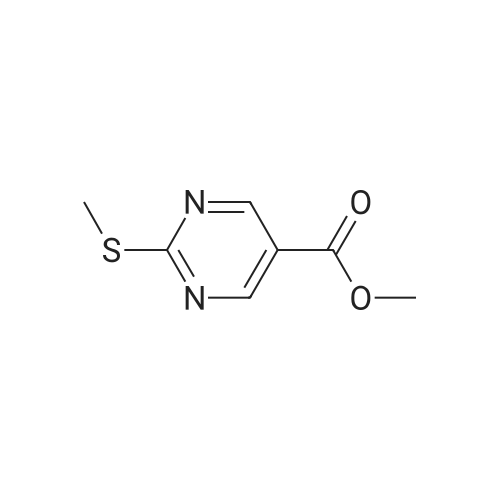
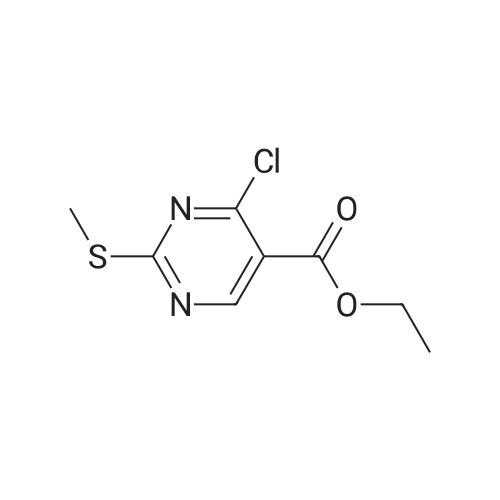
| 51% |
With N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; for 72h;Reflux; |
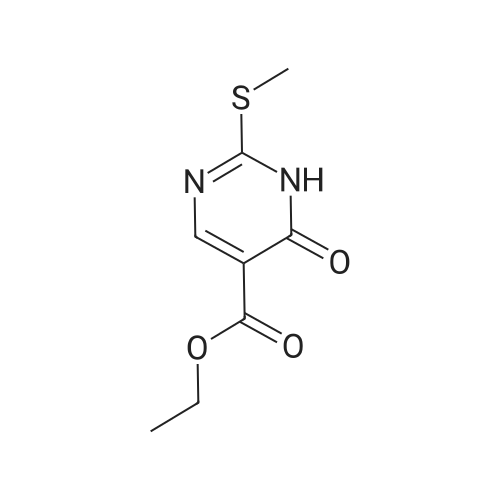
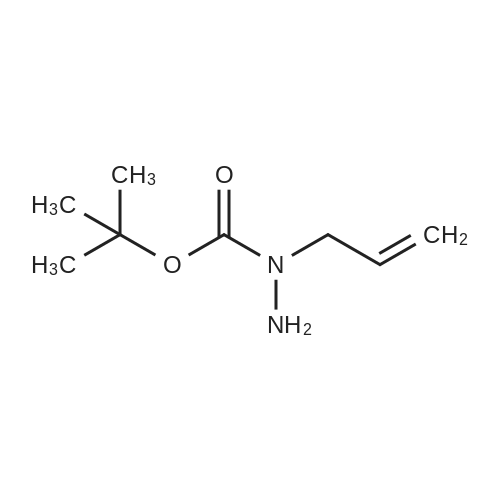
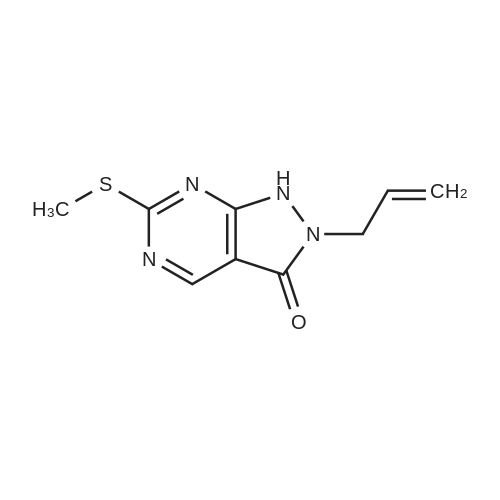
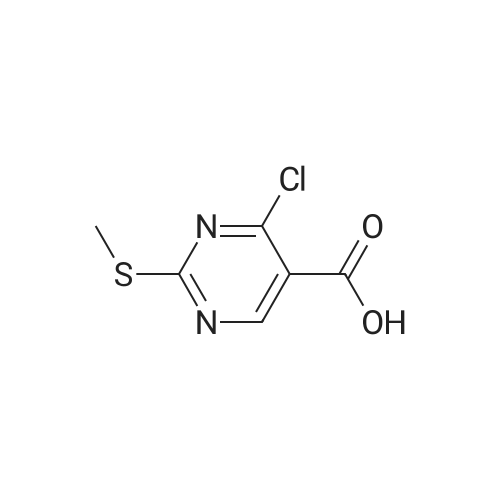
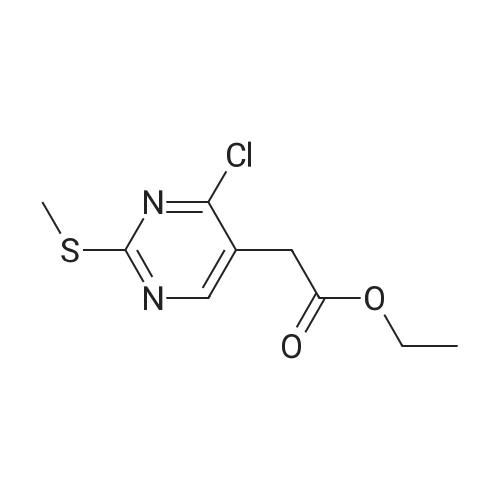
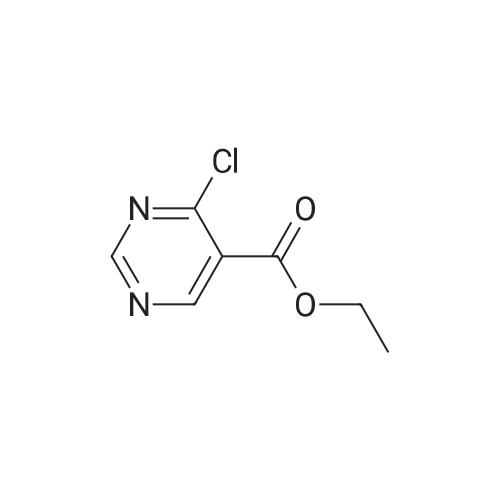
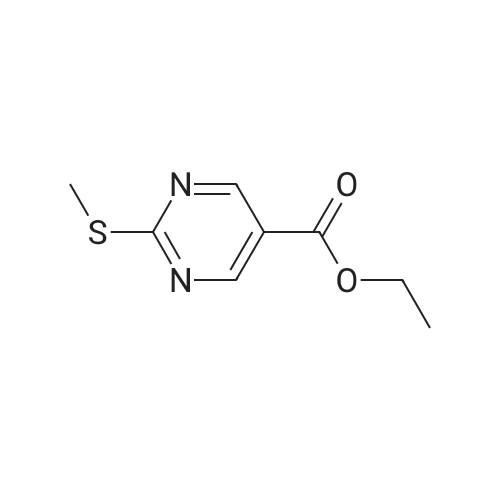
DIPEA (20.8 ml, 120 mmol) and allyl hydrazine 5 (8.23 g, 47.8 mmol) were added to a solution of ethyl 4-chloro-2-methylthio- 5-pyrimidinecarboxylate (6; 1 1.1 g, 47.8 mmol) in THF (150 ml). The reaction mixture was heated at reflux for 72 h, before being concentrated in vacuo. Et20 (50 ml) was added to the residue, and the resultant precipitate was collected by filtration. The filtrate was evaporated to dryness, and the residue was cooled in an ice bath, after which TFA (40 ml) was added. The resultant solution was stirred at RT for 1 h, followed by 70 C for 1 h. The solvent was removed in vacuo and the residue was dissolved in EtOH (50 ml) and cooled in an ice bath, after which 6M NaOH (75 ml) was added. The resultant solution was stirred at RT for 15 min, before 32 being acidified via the addition of cone. HCI (40 ml). The orange solution was evaporated to dryness and the resultant residue was partitioned between chloroform (100 ml) and water (100 ml), and the organic phase was washed with brine (50 ml), dried (Mg2S04), concentrated in vacuo, and triturated with hexanes. The solid precipitate was washed with EtOH and Et20, before being dried under vacuum to give the target compound as a yellow solid (5.44 g, 24.5 mmol, 51 %). Rf 0.45 (9:1 DCM:MeOH); M.p. 125- 128 C; IR (cm-1 ) 3032, 2979, 2926, 2659, 1656, 161 5, 1 566, 1 514; 1 H NMR (400 M Hz, DMSO-d6)2.53 (3H, s, -SCH3), 4.38 (2H, dapp, J = 5.2 Hz, N2-CH2), 5.06-5.20 (2H, m, allyl C-Hcis/trans), 5.87 (1 H, ddt, J = 17.2, 10.5, 5.3 Hz, alkene C-H), 8.67 (1 H, s, H-4), 12.65 (1 H, -1 ); MS [M + H] + m/z 223.1. |
|
|
260 mL of N,N-diisopropylethylamine and 106 g of the hydrazine obtained in the above 1 were added to tetrahydrofuran (1.5 L) solution of 142 g of ethyl 4-chloro-2-(methylthio)pyridine-5-carboxylate, and stirred with heating under reflux for 18 hours. After cooled to room temperature, the reaction solution was evaporated under reduced pressure, and 500 mL of diethyl ether was added to the residue, and the precipitated solid was separated through filtration. The filtrate was evaporated under reduced pressure, the residue was cooled in an ice bath, 400 mL of trifluoroacetic acid was gradually added thereto, and stirred at room temperature for 1 hour and then at 70 C. for 1 hour. The reaction solution was evaporated under reduced pressure, 500 mL of ethanol was added thereto and cooled in an ice bath, and 1.0 L of 6 N sodium hydroxide solution was added thereto and stirred at room temperature for 15 minutes. Cooled in an ice bath, the reaction solution was made acidic with 400 mL of concentrated hydrochloric acid, and then evaporated under reduced pressure. The residue was partitioned in chloroform and water, and the chloroform layer was extracted, washed with saturated saline water, and dried with anhydrous sodium sulfate. The solvent was evaporated away under reduced pressure, and the formed yellow solid was taken out through filtration, washed with ethanol and diethyl ether, and dried to obtain 99.1 g of the entitled compound as a yellow solid.1H-NMR (400 MHz, DMSO-d6) ?: 8.66 (1.0H, brs), 5.83 (1.0H, ddt, J=17.1, 9.8, 5.4 Hz), 5.13 (1.0H, d, J=9.8 Hz), 5.06 (1.0H, d, J=17.1 Hz), 4.34 (2.0H, d, J=5.4 Hz), 2.51 (3.0H, s).ESI-MS Found: m/z[M+H]+ 223.3. |
|
|
2) Production of 2-allyl-6-(methylthio)-l ,2-dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one:260 mL of N,N-diisopropylethylamine and 106 g of the hydrazine obtained in the above 1 were added to tetrahydrofuran (1.5 L) solution of 142 g of ethyl 4-chloro-2- (methylthio)pyridine-5-carboxylate, and stirred with heating under reflux for 18 hours. After cooled to room temperature, the reaction solution was evaporated under reduced pressure, and 500 mL of diethyl ether was added to the residue, and the precipitated solid was separated through filtration. The filtrate was evaporated under reduced pressure, the residue was cooled in an ice bath, 400 mL of trifluoroacetic acid was gradually added thereto, and stirred at room temperature for 1 hour and then at 70C for 1 hour. The reaction solution was evaporated under reduced pressure, 500 mL of ethanol was added thereto and cooled in an ice bath, and 1.0 L of 6 N sodium hydroxide solution was added thereto and stirred at room temperature for 15 minutes. Cooled in an ice bath, the reaction solution was made acidic with 400 mL of concentrated hydrochloric acid, and then evaporated under reduced pressure. The residue was partitioned in chloroform and water, and the chloroform layer was extracted, washed with saturated saline water, and dried with anhydrous sodium sulfate. The solvent was evaporated away under reduced pressure, and the formed yellow solid was taken out through filtration, washed with ethanol and diethyl ether, and dried to obtain 99.1 g of the entitled compound as a yellow solid. iH-NMR (400 MHz, DMSO-d6) delta: 8.66 (1.0H, brs), 5.83 (1.0H, ddt, J=17.1, 9.8, 5.4 Hz), 5.13(l.OH, d, J=9.8 Hz), 5.06 (1.0H, d, J=I 7.1 Hz), 4.34 (2.0H, d, J=5.4 Hz), 2.51 (3.0H, s). ESI-MS Found: m/z[M+H]+ 223.3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping