|
|
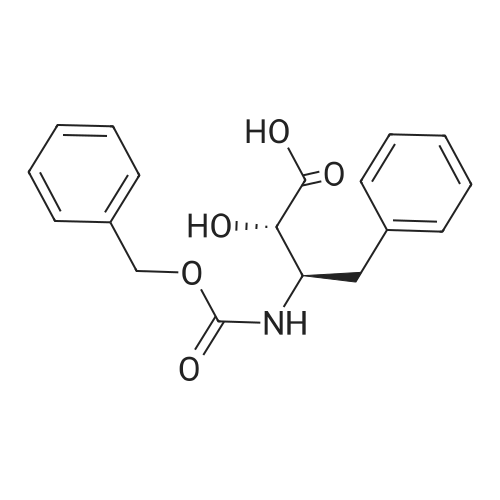
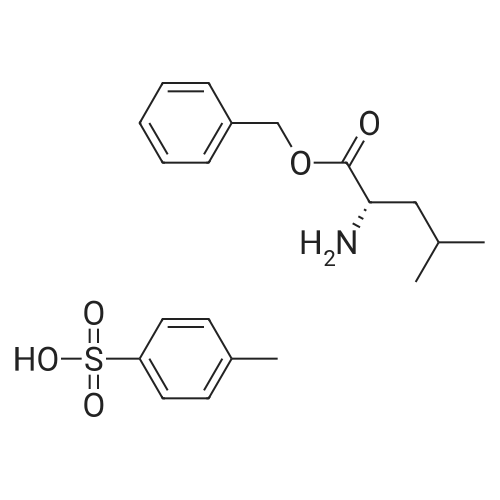
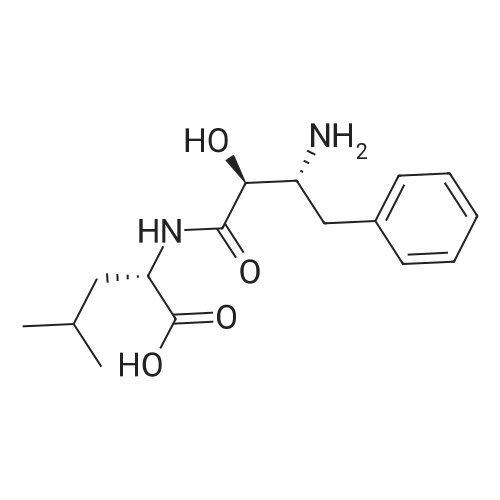
1000 mL of tetrahydrofuran was added to a three-necked flask, and stirring was started, and Z-AHPA 100 g, L-leucine benzyl p-toluenesulfonate 131.5 g, and 1-hydroxybenzotriazole (HOBt) 48.5 g were sequentially added, and then the temperature was lowered to 0±2 C.33.6 g of triethylamine and 74.5 g of dicyclohexylcarbodiimide (DCC) were added. After the addition, the temperature was controlled at 12±2 C, and the reaction was stirred for 20 to 24 hours. After the reaction was completed, the reaction solution was filtered.The filtrate was concentrated under reduced pressure to dryness crystals eluted eluted eluted elute The above ethyl acetate solution was washed with 650 mL of 0.5 mol/L hydrochloric acid, 650 mL of purified water, 650 mL of a saturated sodium hydrogencarbonate solution, and 650 mL of a saturated sodium chloride solution. The organic layer was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure at 50 C to dryness without distillation; 270 mL of ethyl acetate was added, heated to 70±2C, stirred until dissolved, cooled to 5±2C, stirred to crystallization in hours. Filter and filter cake twice with 100 mL of ethyl acetate / petroleum ether (1:2). After filtration, the cake was taken out and dried to obtain 140 g of a condensate product.The hydrogen gas was passed to the pressure inside the reactor at 1 MPa, and the stirring was started, and hydrogen was added until hydrogen was almost no longer absorbed (the pressure did not change within half an hour in the autoclave). Drain, release the reaction solution; reaction kettle with glacial acetic acid 100mL After washing once, the reaction solution was filtered, the filter cake was washed with acetic acid, and the filtrate was concentrated under reduced pressure at 60 C; while no fraction was distilled off, acetone 530 mL was added and stirred at room temperature for 2 to 3 hours. The filter cake was washed with acetone, and dried to give crude ubenimex 85g, yield 60.7%.ii refinement of ubenimex 900 mL of a 1 mol/L hydrochloric acid solution was placed in a container, heated to 55 C, and a total of 85 g of crude ubenimex was added, and stirred until dissolved. 3.30 g of activated carbon was added, and the mixture was decolorized by stirring for 15 minutes, and filtered. The filtrate was adjusted with ammonia water to pH=5.5, cooled to 0-5 C, stirred for 2 hours, filtered, and the filter cake was washed three times with 250 mL of purified water. The filter cake was transferred to a three-necked flask and added with C.The ketone was 900 mL and stirred at room temperature for 4 hours. After filtration, the filter cake was washed twice with acetone, 80 mL each time, and dried to obtain 72.2 g of ubenimex. The liquid chromatogram of ubenimex is shown in Figure 1.iii Collection of compounds of formula II The mother liquor which was filtered three times after the crystallization was collected, concentrated under reduced pressure to about 200 ml of dryness, and the pH of the solution was adjusted to 5.5, and crystals were precipitated. The temperature was lowered to 0-5 C, and the mixture was further stirred for 2 hours, filtered, and the filter cake was washed with purified water. The combined mother liquid was concentrated under reduced pressure to about 60 ml, and the solution was adjusted to pH 5.5, and the mixture was stirred and crystallized. Cool down to 0-5 C, continue to stir and crystallize for 2 hours, filter, motherThe liquid was concentrated to dryness under reduced pressure, washed with water and dried to give 3 g of solid. The purity of the target impurity compound was 35%. The chromatographic separation of the target impurity product was 0.05 g. The purity of the product was 98.2% by mass spectrometry. The mass spectrometry ESI(+) was 309.14 (M+1). The mass spectrum of the LC/MS test is shown in Figure 2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping