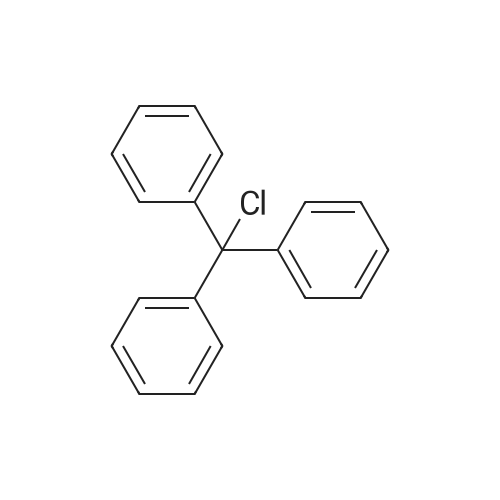
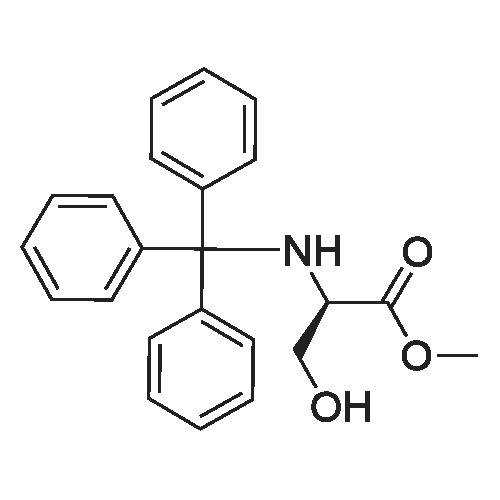
| 92% |
With triethylamine; In dichloromethane; at -5 - 20℃; |
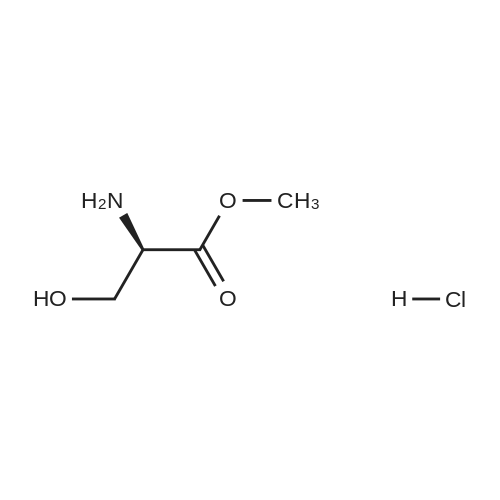
A compound of D- serine methyl ester hydrochloride (1000g, 6.45mol, 1eq) was dissolved in 5L of dichloromethane, was added triethylamine (1955g, 19.35mol, 3eq), maintaining the temperature at -5 ~ 5 , min after the batch was added triphenylmethyl chloride (2152g, 7.74mol, 1.2eq) was stirred at room temperature until the reaction is complete, water was added quenched liquid separation, the organic phase was washed with hydrochloric acid, TLC testing to confirm no triethylamine dried over anhydrous sodium sulfate, the solvent was concentrated to give a white solid, a -D- N- trityl-serine methyl ester. Yield 92%. |
|
With triethylamine; In dichloromethane; at 0℃; for 12h;Inert atmosphere; |
General procedure: To a suspension of (S)-serine methyl ester hydrochloride (0.50 g, 3.2 mmol) in DCM (7 ml) at 0 C, under a nitrogen atmosphere, was added dropwise triethylamine (1.0 ml, 7.1 mmol), followed by a solution of triphenylmethyl chloride (0.91 g, 3.2 mmol) in DCM (2 ml). After stirring at 0 C for 12 h the white precipitate was filtered and the filtrate was concentrated in vacuo to yield a white solid which was dissolved in ethyl acetate (10 ml), washed with 1 M aqueou NaHCO3 solution (10 ml), 10% aqueous citric acid solution (10 ml) and water (10 ml). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo giving a white solid. Purification by flash column chromatography on silica gel in petroleum ether:ethyl acetate (4:1), gave N-trityl-(S)-serine methyl ester as a white solid (0.99 g, 86%). |
|
With triethylamine; In dichloromethane; at 0℃; for 12h;Inert atmosphere; |
General procedure: N-Trityl-(S)-serine methylester. To a suspension of (S)-serine methyl ester hydrochloride (0.50 g, 3.2mmol) in DCM (7 ml) at 0 oC, under a nitrogen atmosphere, was added dropwise triethylamine (1.0 ml, 7.1 mmol), followed by a solution of triphenylmethyl chloride (0.91 g, 3.2 mmol) in DCM (2 ml). After stirring at 0 oC for 12 h the white precipitate was filtered and the filtrate was concentrated in vacuo to yield a white solid which was dissolved in ethyl acetate (10 ml), washed with 1 M aqueous NaHCO3 solution (10 ml), 10% aqueous citric acid solution (10 ml) and water (10 ml). The organic layer was dried over anhydrous magnesium sulfate and concentrated in vacuo giving a white solid. Purification by flash column chromatography on silica gel in petroleum ether:ethyl acetate(4:1), gave N-trityl-(S)-serine methyl ester as a white solid (0.99 g, 86%). |
|
With triethylamine; In chloroform; at 0 - 20℃; for 1h; |
Example 1: Preparation of (R)-4-[(R)-3-amino-4-(2,4,5-trifluorophenvDbutanoyll- 3-(t-butoxymethyl)piperazin-2-one hydrochloride; Step 1: Preparation of (R)-methyl l-tritylaziridine-2-carboxylate; 200 g of D-serine methyl ester hydrochloride was added to 1.8 L of chloroform, and the reaction solution was cooled to 0C, to which 448 mL of triethylamine was then slowly added. 358.4 g of trityl chloride was slowly added to the reaction mixture which was then stirred for 1 hour. The reaction mixture was warmed to room temperature, and 1 L of chloroform was added thereto, followed by washing with 2.5 L of water. The organic layer was dried over magnesium sulfate and cooled to 0C, to which 484 mL of triethylamine and 15.7 g of 4- methylaminopyridine were then sequentially and slowly added. The reaction mixture was stirred for 5 min and 139 mL of methane sulfonyl chloride was slowly added thereto. The reaction mixture was warmed to room temperature, stirred for another 4 hours and then refluxed for 12 hours. The reaction mixture was cooled to room temperature, and washed with 4 L of water and then 3 L of brine. The organic layer was dried over magnesium sulfate and <n="17"/>concentrated to dryness under reduced pressure. 3 L of ethanol was added to the resulting residue which was then stirred. The resulting solids were filtered to afford 329 g of the title compound.IH NMR (400 MHz, CDC13): 7.42 to 7.49(m, 6H), 7.18 to 7.32(m, 9H), 7.68(s, IH),3.74(s, 3H), 2.24(m, IH), 1.87(m, IH), and 1.40(m, IH) |
|
With triethylamine; In dichloromethane; at 0℃; for 24h;Inert atmosphere; |
(ft)-Methyl 3-Hydroxy-2-(λ/-tritylamino)propanoate (S. Bhatia and J. Hajdu, Tetrahedron Lett. 1998, 29, 31-34).; To a solution of D-serine methyl ester hydrochloride (20.00 g, 0.13 mol) and Et3N (35.82 mL, 0.26 mol) in CH2CI2 (80 mL) at 0 0C, was added in one portion a solution of TrCI (36.53 g, 0.13 mol) in CH2CI2 (80 mL). The mixture was allowed to stir at 0 0C (24 h) under Ar and then successively washed with 10% aqueous citric acid (120 mL) and saturated aqueous brine (120 mL). The organic layer was dried (Na2SO4) and evaporated in vacuo to yield 45.20 g (97%) of crude desired product as a pale yellow crystalline solid. The product was used for next step without further purification: Rf = 0.50 (1/1 EtOAc/hexanes); 1H NMR (CDCI3) δ 2.29 (br s, 1 H), 2.98 (br s, 1 H), 3.29 (S, OCH3), 3.51-3.60 (m, CHH'OH, CH), 3.64-3.74 (m, CHH'OH), 7.16-7.30 (m, 9 PhH), 7.47-7.50 (m, 6 PhH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping