| 100% |
|
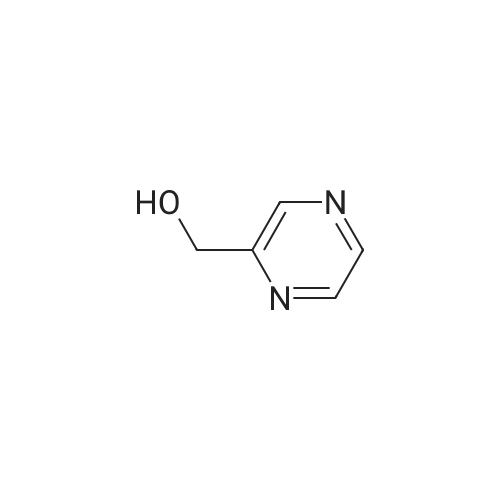
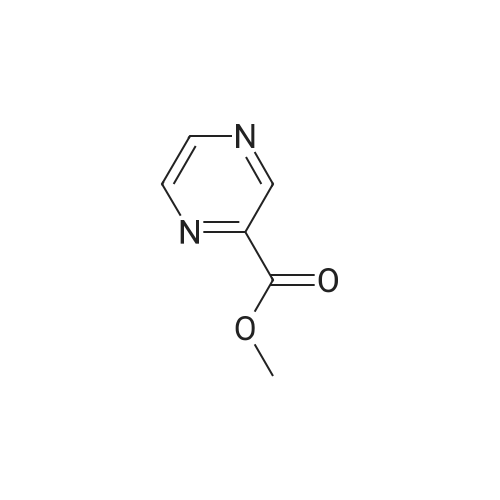
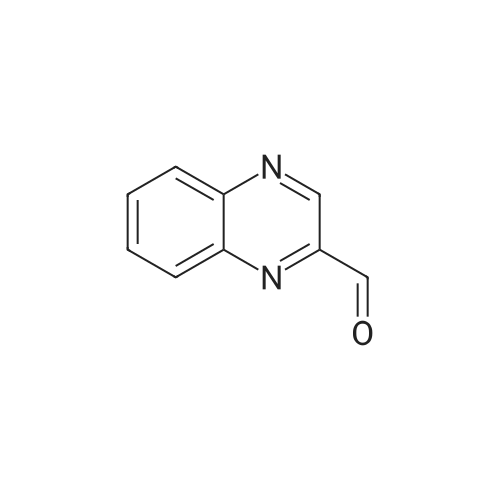
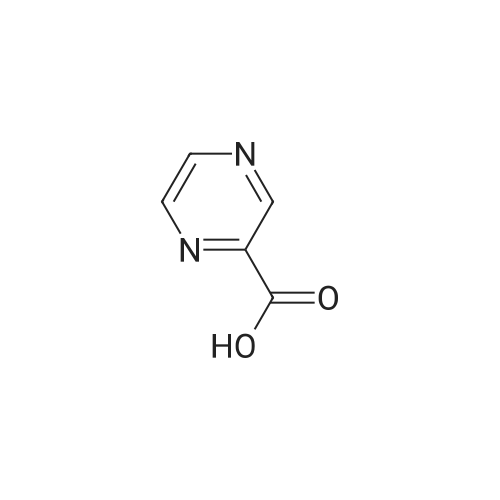
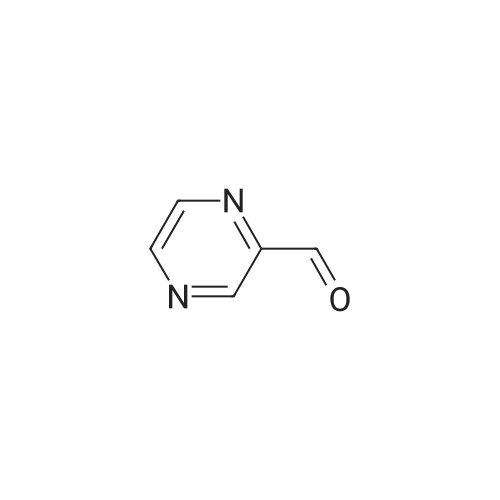
Method 54; Pvrazine-2-carboxaldehyde oxime A IN solution of lithium aluminium hydride in THF (73. 8ml, 73.8mmol) was added to a suspension of methyl pyrazine-2-carboxylate (20g, 145mmol) in anhydrous THF (300. 0ml) at-78°C keeping the reaction temperature below-72°C. On completion of addition the reaction mixture was left to stir at-78°C for a further 20 minutes and then quenched with glacial acetic acid (20. 0ml). The resulting mixture was warmed to room temperature and the volatiles removed by evaporation. The residue was dissolved in 3N hydrochloric acid (116ml) and extracted with DCM. The extracts were combined, washed with saturated aqueous sodium hydrogen carbonate solution and the solvent evaporated. The residue was purified by chromatography on silica gel eluting with DCM/diethylether (100: 0 then 80: 20 and then 0: 100) to give pyrazine-2-carboxaldehyde (15.67g, 100percent). This was immediately dissolved in chloroform (200ml) cooled to 0°C and hydroxylamine mono-hydrochloride (11. 02g, 159. 5mmol) and triethylamine (24. 2ml, 117. 4mmol) were added. The reaction mixture was then stirred at ambient temperature for 0.5 hour, and the solvent removed by evaporation. The residue suspended in diethylether (500ml) and the insolubles removed by filtration. The filtrate was evaporated and the residue purified by chromatography eluting with DCM/diethylether (100: 0 then 80: 20 and then 0: 100) to give the title compound (5. 5g, 31percent) as a solid. NMR (DMSO-d6) : 8.15 (s, 1H), 8.62 (dd, 2H), 8. 99 (s, 1H). |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at -78℃; for 1h;Inert atmosphere; |
A solution of the product of Example 83A (6.91 g, 50 mmol) in THF (Aldrich, anhydrous, 150 mL) was cooled down to -78° C. and a solution of LiAlH4 (Aldrich, 1.898 g, 50.0 mmol) in THF (50 mL) was added slowly via an additional funnel. The mixture was stirred at -78° C. under N2 for 1 hour, and then it was then carefully and slowly quenched with HOAc (Aldrich, 10 mL) at -70° C. The mixture was slowly warmed up to ambient temperature and stirred for 10 hours. After being concentrated, the residue was stirred with HCl (2N, 15 mL) in CH2Cl2 (300 mL) for 20 minutes and then filtered through diatomaceous earth to remove solid inorganic salt. The organic filtrate solution was concentrated and the residue was dissolved in EtOAc (100 mL) and filtered through diatomaceous earth again. The organic filtrate solution was concentrated to give the titled compound. 1H NMR (300 MHz, DMSO-d6) delta 8.91 (dd, 1H), 8.94 (d, 1H), 9.12 (d, J=1.6 Hz, 1H), 10.08 (s, 1H) ppm; MS (DCI/NH3) m/z 109 (M+H)+. |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at -70℃; for 0.666667h; |
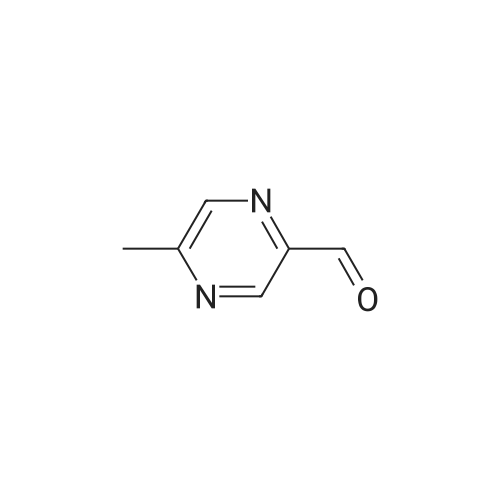
LiAlH4 (4g, 100 mMol) was slowly added to a solution of pyrazinecarboxylic acid methyl ester (12. 8g, 100 mMol) in tetrahydrofuran (400 mL) at-70° C. The mixture was stirred at this temperature for 40 min, followed by neutralisation with 20 mL of glacial acetic acid. The solvent was evaporated in vacuo and the residue was partitioned between 30 mL of 2N HCL and dichloromethane. A large amount of brown solid precipitated which was filtered off. The dichloromethane layer was passed through a pad of silica, the solvent was evaporated to give the pyrazine 2-carboxaldehyde (1.4g) as a yellow oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping