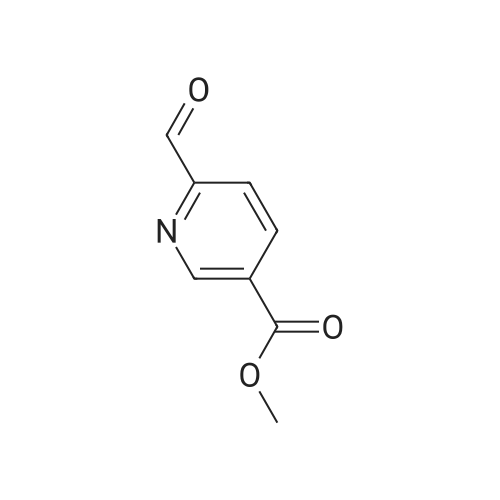
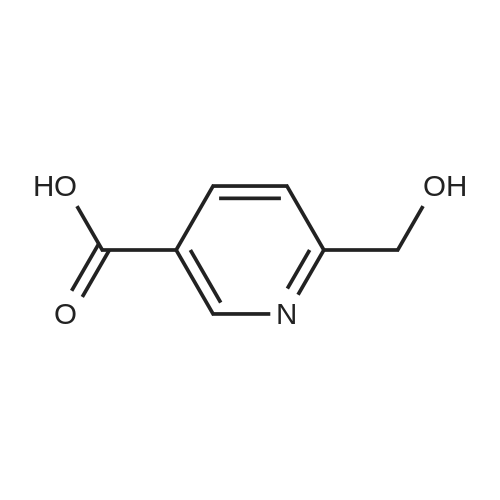
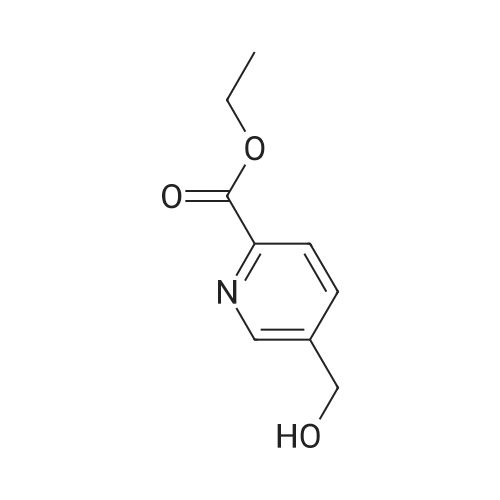
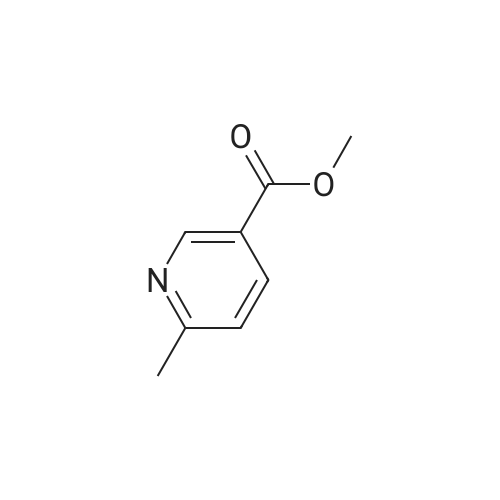
| 85.6% |
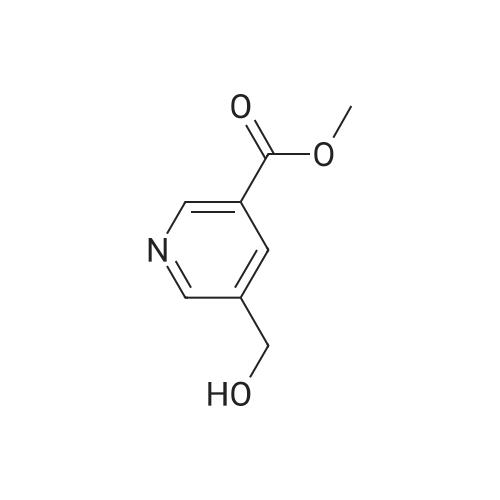
With Dess-Martin periodane; In dichloromethane; |
The A solution of compound 25 (1.0 g, 6. Ommol)Dissolved in dichloromethane (10 mL)in,Add a Dess-Martin periodinane(3.0 g, 7.2 mmo 1)TLC to track the degree of completion of the assay,After the reaction is completed,Water extraction, ethyl acetate extraction,Concentration gave compound 26 (0.6 g, 85.6% |
| 71.4% |
|
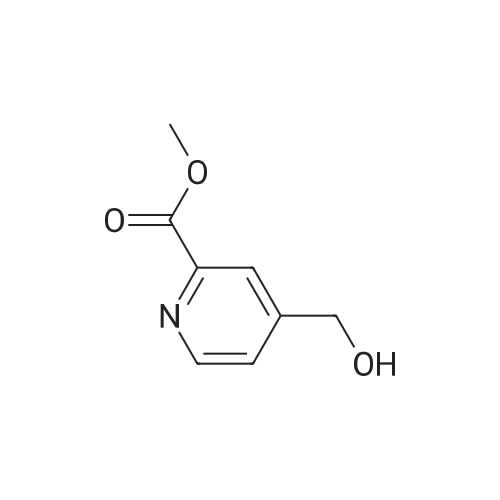
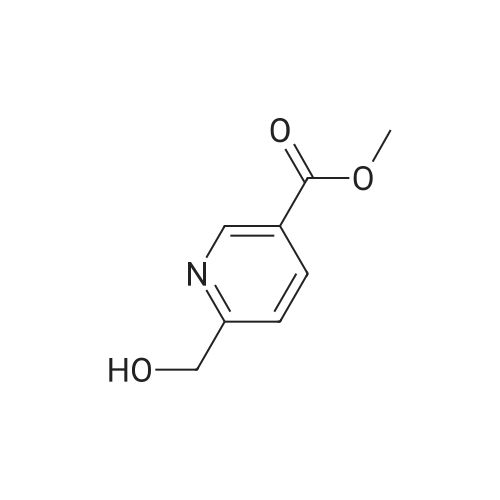
To a solution of oxalyl chloride (2.0 M in dichloromethane, 8.97 mL) in dichloromethane (35 mL) at -78 C was added (methylsulfinyl)methane (2.55 mL, 35.9 mmol) dropwise. This mixture was allowed to stir at -78 C for 10 minutes then methyl 6-(hydroxymethyl)nicotinate (2.0 g, 11.96 mmol, Combi-Blocks) was added. The mixture was allowed to stir for an additional 15 minutes and triethylamine (6.67 mL, 47.9 mmol) was added, and the mixture was stirred for an additional 15 minutes at -78 C. The dry ice-acetone bath was then replaced with an ice-water bath, and the mixture was allowed to stir for 20 minutes. The mixture was quenched with saturated, aqueous NaHC( (15 mL) and the layers were separated. The aqueous layer was extracted with dichlorome thane (3 x 10 mL), and the combined organic fractions were dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified via column chromatography (Si(, 50% ethyl acetate/heptanes) to give the title compound (1.41 g, 8.54 mmol, 71.4% yield). MS (ESI+) m/z 166 (M+H)+. |
|
With Dess-Martin periodane; In dichloromethane; for 3h; |
Dess-Martin reagent (207 mg, 0.49 mmol) was added to a stirring solution of 6- hydroxymethyl-nicotinic acid methyl ester (55 mg, 0.33 mmol) in DCM (17 mL). After stirring for 3 h the solvent was removed in vacuo. The residue was purified by flash chromatography on silica gel (elution with n-hexane/EA 4:1) to give the title compound.GCMS (m/z): 165.2 |
|
With manganese(IV) oxide; In dichloromethane; at 20℃; for 4h; |
A mixture of methyl 6-( ydroxymethyi)nicotinate (7 g, 37 mmol) and Mn02 (32,3 g,372 mmoi) in DCM ( 200 mL ) was stirred at 20 Q for 4 hours. The mixture was filtered and the filtrate was concentrated. The residue was purified by column chromatography on silica gel(PE/EtOAc = 5/i) to afford methyl 6-formylnicotinate. MS-ESI (m/z): 166.2 (M+i) + (LC-.MSmethod D; Ret. time: 0.36 mm). |
|
With manganese(IV) oxide; In dichloromethane; at 20℃; |
Intermediate 11-14 Step 1 : methyl 6-formylnicotinate To a solution of methyl 6-(hydroxymethyl)nicotinate (30 g, 179.6 mmol) in DCM (500 mL) was added MnC>2 (93.8 g, 1077.8 mmol). The mixture was stirred at room temperture overnight. The reaction mixture was filtered and concentrated. The residue was purified by column chromatography on silica gel eluted with PE/EA = 10/1 to give methyl 6-formylnicotinate. XH NMR (400 MHz, CDC13) delta ppm 3.98 (d, J= 0.78 Hz, 3 H), 8.01 (d, J= 8.0 Hz, 1 H), 8.40 - 8.49 (m, 1 H), 9.29 - 9.38 (m, 1 H), 10.11 (s, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping