| 97% |
With triethylamine; In N,N-dimethyl-formamide; at 20℃; |
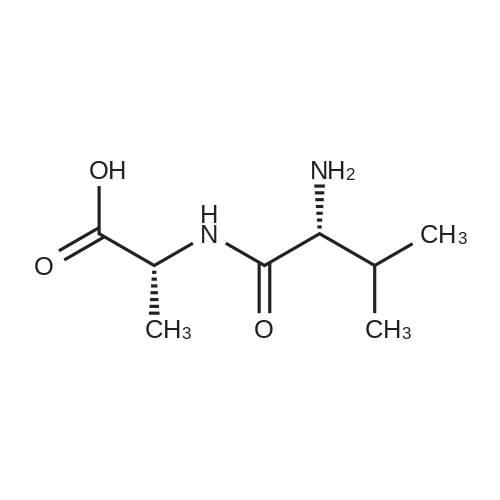
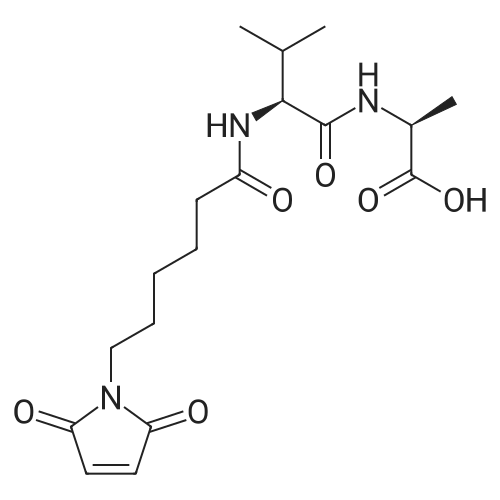
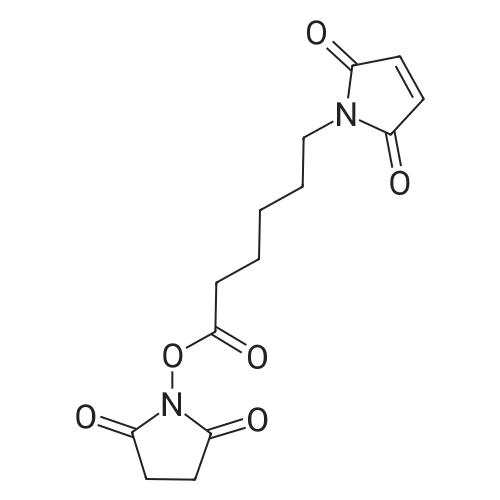
150 mg (0.797 mmol) of <strong>[1115-74-8]L-valyl-L-alanine</strong> and 246 mg (0.797 mmol) of 1-{6-[(2,5-dioxopyrrolidin-1-yl)oxy]-6-oxohexyl}-1H-pyrrole-2,5-dione were dissolved in 4.0 ml of dimethylformamide, and 0.220 ml (1.6 mmol) of triethylamine was added. The reaction mixture was stirred at RT overnight. The reaction mixture was purified directly by preparative RP-HPLC (column: Reprosil 250*30; 10mu, flow rate; 50 ml/min, MeCN/water). The solvents were evaporated under reduced pressure and the residue was dried under high vacuum. This gave 302 mg (97% of theory) of N-[6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl) hexanoyl]-<strong>[1115-74-8]L-valyl-L-alanine</strong>. LC-MS (Method 12): Rt=1.02 min; MS (ESIpos): m/z=382 (M+H)+. 1H-NMR (400 MHz, DMSO-d6): delta [ppm]=0.82 (dd, 6H), 1.17 (m, 2H), 1.27 (d, 3H), 1.48 (m, 4H), 1.94 (m, 1H), 2.13 (m, 2H), 3.38 (t, 2H), 4.17 (m, 2H), 7.00 (s, 2H), 7.75 (d, 1H), 8.19 (d, 1H). |
| 70% |
With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃;Inert atmosphere; |
Maeilimidocaproy. N-hydroxysuccinimide (1.619 g, 5.25 mmo., 105 eq.) and H-Val-Aia-OH (0,941 g, 5 mmol, 1 eq.) were placed in a 25 ml recovery fiask with a stir bar and the flask was flushed with nitrogen, DMF (4,7 mL) was added and the resulting white siurry was stirred. DIPEA (0.87 mL, 5 mmol, 1 eq) was added and the mixture was allowed to stir at room temperature overnight. The mixture was coo.ed in an ice/water bath and 2M HCI (3 mL, 6 mmol) was added dropwise. The viscous mixture was transferred to a separator/ funnel and the reaction vessel rinsed with sat. NaCl (7 mL), EtOAc (10 mL), sat NaCl {10 mL) and EtOAc (5 mL). After separation of the aqueous phase, it was extracted with additionai EtOAc {2 x 15 mL). The combined organic extracts were washed with sat NaCi {4 x 15 mL), until the washings were pH ~3,5. The organic extracts were dried over Na2S04, filtered and concentrated under reduced pressure to give crude 5 as a white solid (2,1 2 g, 114% crude yield). Crude 5 was suspended in warm CHjCIa {35 mL) and filtered to remov a fine white solid. The solids wer rinsed with additional CHjCi2 (3 mL). Toiuene {5mL) was added and the mixture was cooled in an ice/water bath, which resulted in a thick siurry. The solids were collected by filtration, washed with a cold mixture of CHjC (12 mL) and toluene (2 mL) and dried by puiilng air through the sample overnight to give 5 as a White solid (1 ,327 g, 70% yield). TLC: Rf = 0.26, 10% MeOH in CH2CI2. 1 H NMR (CDCI3)(ppm) 0.95 {d, J - 17 Hz, 3H), 0.98 (d, J - 17 Hz, 3H), 1.30 (m, 2H), 1.40 (d, J = 17 Hz, 3H), 1.61 (m, 4H), 2.06 (m, 1 H), 2.25 (dt, J = 4, 19 Hz, 2H). 3.35 (s, 1 H), 3.49 (t, J - 17 Hz,2H), 4.20 (d, J = 18 Hz, 1 H), 4.38 (m, 1 H), 6.80 {s, 2H). Analytical HPLC (0.1% formic acid): tR 9.05 min. LC-MS: tR 11.17 min, m/z (ES+) found 381.9 (M+H)+, m/z (ES-) found 379.9 (M-H)-. |
| 52% |
In N,N-dimethyl-formamide; at 20℃; for 48h; |
(a) (S)-2-((S)-2-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanamido)-3- methylbutanamido)propanoic acid (23)A suspension of dipeptide (22) (0.1 g, 0.54 mmol, 1 eq.) and 6-maleimidohexanoic acid succinimide ester (0.165 g, 0.54 mmol, 1 eq.) in anhydrous DMF (5 mL) was stirred at room temperature for 24 hours at which time LCMS indicated 50% conversion to a new product. The reaction mixture was diluted with anhydrous DMF (5 mL) and the reaction was allowed to continue for a further 24 hours. The solvent was evaporated under reduced pressure to give a colourless residue. Diethyl ether (60 mL) was added and the mixture was sonicated for 5 min, the ether was decanted and the process was repeated (x 2). The final ethereal portion was filtered to isolate the product (23) as a white powder which was dried under vacuum (0.105 g, 52%). Analytical Data: RT 2.28 min; MS (ES+) m/z (relative intensity) 382 ([M + H]+ , 90), MS (ES") m/z (relative intensity) 380 ([M - H])-, 100). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping