Alternatived Products of [ 5332-25-2 ]
Product Details of [ 5332-25-2 ]
| CAS No. : | 5332-25-2 |
MDL No. : | MFCD00024023 |
| Formula : |
C9H6BrN
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | IFIHYLCUKYCKRH-UHFFFAOYSA-N |
| M.W : |
208.05
|
Pubchem ID : | 79243 |
| Synonyms : |
6-Br-Quinoline
|
Calculated chemistry of [ 5332-25-2 ] Expand+
Physicochemical Properties
| Num. heavy atoms : |
11 |
| Num. arom. heavy atoms : |
10 |
| Fraction Csp3 : |
0.0 |
| Num. rotatable bonds : |
0 |
| Num. H-bond acceptors : |
1.0 |
| Num. H-bond donors : |
0.0 |
| Molar Refractivity : |
49.44 |
| TPSA : |
12.89 ?2 |
Pharmacokinetics
| GI absorption : |
High |
| BBB permeant : |
Yes |
| P-gp substrate : |
No |
| CYP1A2 inhibitor : |
Yes |
| CYP2C19 inhibitor : |
No |
| CYP2C9 inhibitor : |
No |
| CYP2D6 inhibitor : |
No |
| CYP3A4 inhibitor : |
No |
| Log Kp (skin permeation) : |
-5.56 cm/s |
Lipophilicity
| Log Po/w (iLOGP) : |
2.19 |
| Log Po/w (XLOGP3) : |
2.83 |
| Log Po/w (WLOGP) : |
3.0 |
| Log Po/w (MLOGP) : |
2.56 |
| Log Po/w (SILICOS-IT) : |
3.17 |
| Consensus Log Po/w : |
2.75 |
Druglikeness
| Lipinski : |
0.0 |
| Ghose : |
None |
| Veber : |
0.0 |
| Egan : |
0.0 |
| Muegge : |
1.0 |
| Bioavailability Score : |
0.55 |
Water Solubility
| Log S (ESOL) : |
-3.59 |
| Solubility : |
0.054 mg/ml ; 0.00026 mol/l |
| Class : |
Soluble |
| Log S (Ali) : |
-2.76 |
| Solubility : |
0.363 mg/ml ; 0.00174 mol/l |
| Class : |
Soluble |
| Log S (SILICOS-IT) : |
-4.56 |
| Solubility : |
0.00574 mg/ml ; 0.0000276 mol/l |
| Class : |
Moderately soluble |
Medicinal Chemistry
| PAINS : |
0.0 alert |
| Brenk : |
0.0 alert |
| Leadlikeness : |
1.0 |
| Synthetic accessibility : |
1.19 |
Application In Synthesis of [ 5332-25-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 5332-25-2 ]
- 1
-
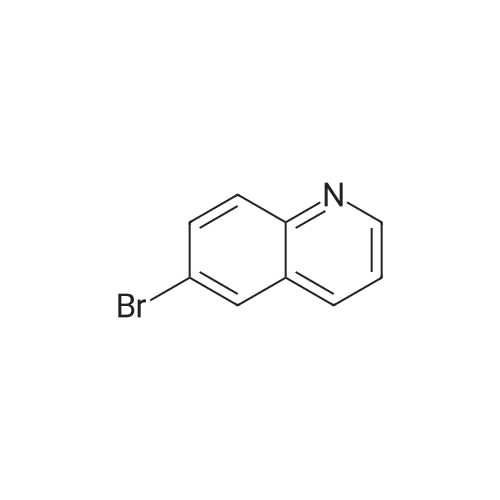 [ 5332-25-2 ]
[ 5332-25-2 ]

-
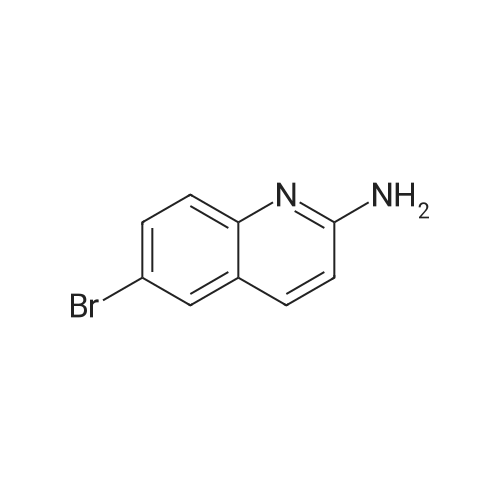 [ 791626-58-9 ]
[ 791626-58-9 ]
Reference:
[1]Patent: US2011/152246,2011,A1
[2]Dyes and Pigments,2013,vol. 99,p. 240 - 249
[3]Journal of Materials Chemistry C,2015,vol. 3,p. 3774 - 3782
[4]Advanced Synthesis and Catalysis,2020,vol. 362,p. 5777 - 5782
- 2
-
 [ 5332-25-2 ]
[ 5332-25-2 ]

-
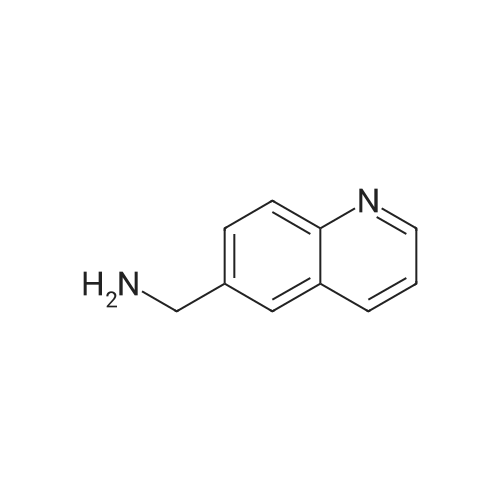 [ 99071-54-2 ]
[ 99071-54-2 ]
- 3
-
 [ 5332-25-2 ]
[ 5332-25-2 ]

-
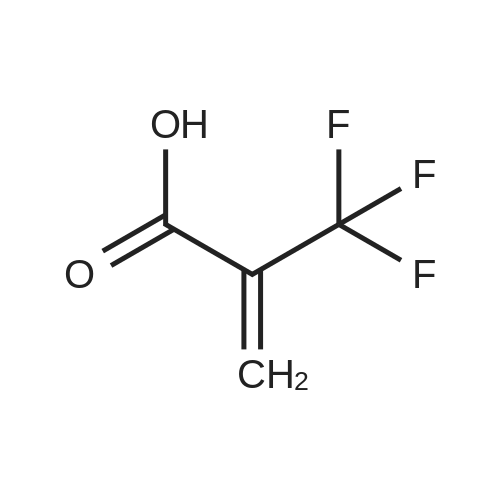 [ 381-98-6 ]
[ 381-98-6 ]

-
6-[(E)-3,3,3-trifluoro-1-propen-1-yl]quinoline
[ No CAS ]
- 4
-
 [ 5332-25-2 ]
[ 5332-25-2 ]

-
 [ 98-80-6 ]
[ 98-80-6 ]

-
 [ 3894-25-5 ]
[ 3894-25-5 ]

-
 [ 861872-54-0 ]
[ 861872-54-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 35 mg; 45 mg |
With ammonium peroxodisulfate; zinc trifluoromethanesulfonate; silver nitrate; In dichloromethane; water; at 20℃; for 4h; |
General procedure: Ammonium persulfate (3 equiv, 342 mg), phenylboronic acid (1.5 equiv, 91 mg), silver nitrate (0.2 equiv, 17 mg), and zinc trifluoromethanesulfonate (0.2 equiv, 36 mg) were combined ina 10 mL round bottom flask. A heterocycle (1 equiv, 0.5 mmol) was then added to the same flask and solvated with water (0.4 mL) and CH2Cl2 (1.6 mL). The resulting mixture was sonicated for 10 sec and placed on a stir plate to stir vigorously at room temperature for 4 h. The reaction was quenched with 28% ammonium hydroxide (2 mL), diluted with water (10 mL), and extracted with CH2Cl2 (3 x 10 mL). The organic layer was dried over sodium sulfate, filtered through cotton, and evaporated en vacuo. The products were purified by column chromatography (SiO2, 5-20% EtOAc/hexanes). Products 3b, 3c, and 3d required further purification by crystallization as the trifluoromethanesulfonic acid salts and recrystallization from THF/hexanes. |
- 5
-
 [ 5332-25-2 ]
[ 5332-25-2 ]

-
 [ 1128-56-9 ]
[ 1128-56-9 ]

-
C18H14N4
[ No CAS ]
- 6
-
 [ 5332-25-2 ]
[ 5332-25-2 ]

-
 [ 100-59-4 ]
[ 100-59-4 ]

-
 [ 3894-25-5 ]
[ 3894-25-5 ]