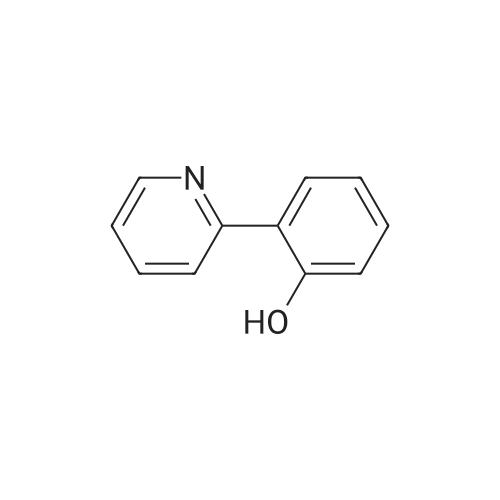
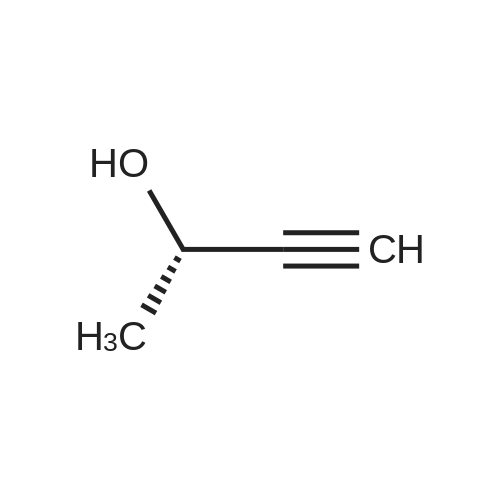
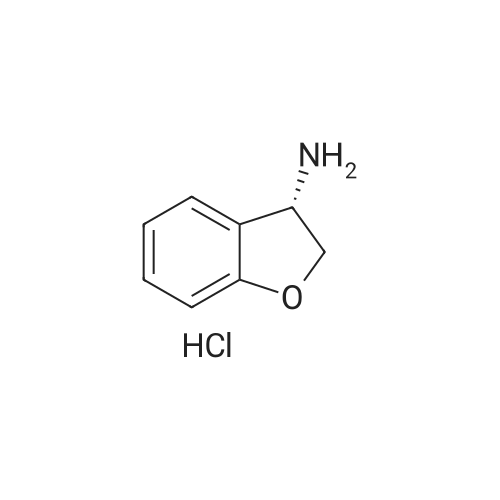
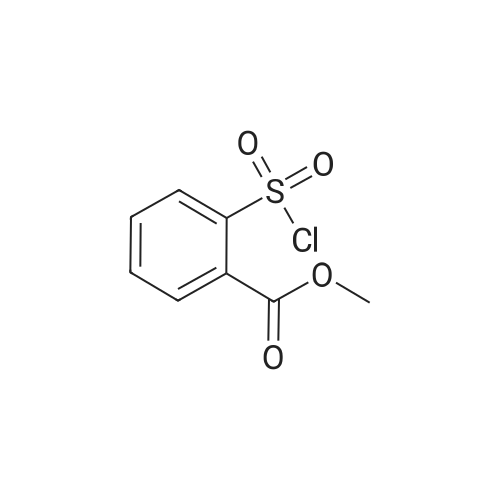
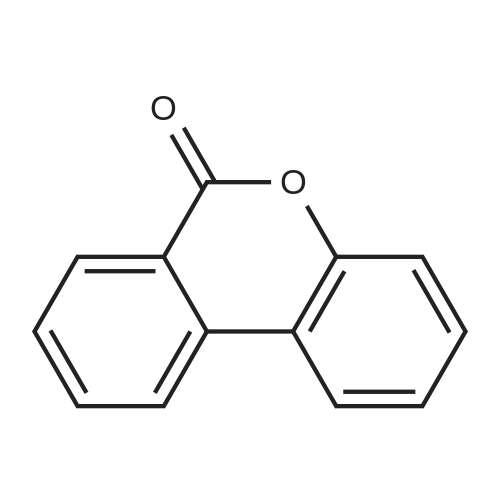
| 79% |
With triethylamine; magnesium chloride; In acetonitrile; at 10 - 72℃; for 2h; |
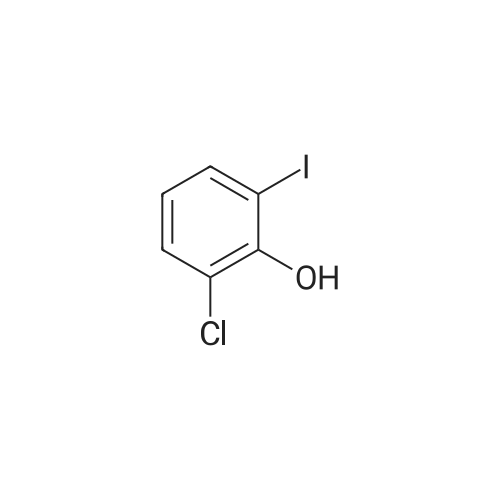
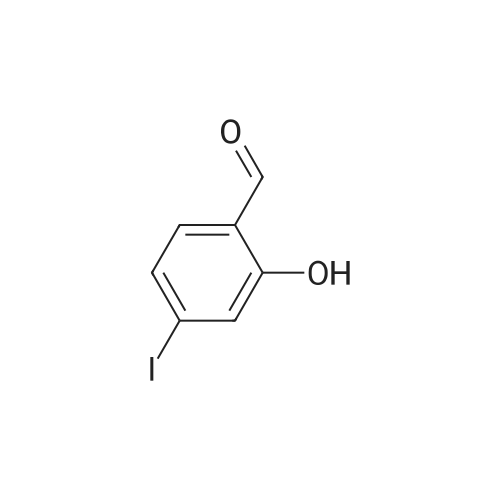
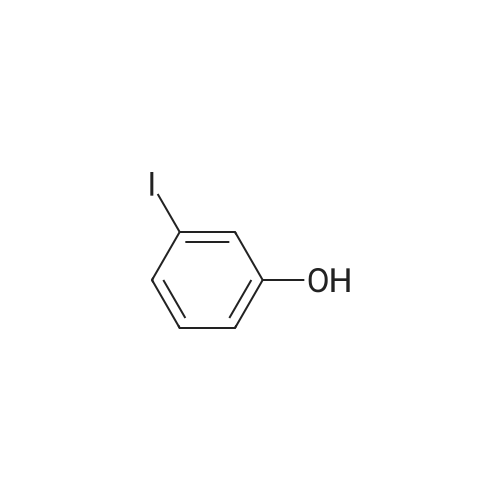
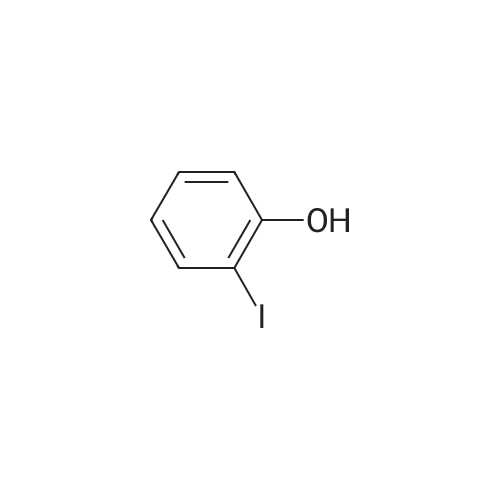
4-(nitrooxy)butyl (2R)-7-benzyl-6-chloro-2-(trifluoromethyl)-2H-chromene-3- carboxylate; Step 1; Preparation of 2-hydroxy-4-iodobenzaldehyde; [0168] To a chilled solution of commercially available 2-iodophenol (30 g, 136 mmole) in ACN was added MgCl2 (19.5 g, 204 mmole) portion-wise while maintaining the temperature below 10 0C, followed by paraformaldehyde (28.6 g, 954 mmole) and TEA (76 mL, 545 mmole) producing a 15 C exotherm. The solution was heated to 72 0C for 2 h. The reaction was cooled to room temperature and poured into Saturated aqueous Ammonium Chloride (500 mL), extracted with ethyl acetate (2 X 150 mL). The combined organic phases were washed with aqueous NaHCO3 solution (2 X 150 mL), aqueous IN HCl solution (2 X 150 mL), and brine (2 X 150 mL), dried over Na2SO4, filtered and concentrated in vacuo. The crude material was subjected to flash chromatography (Silica, 5% Ethyl acetate/ Hexane). Desired fractions were collected and combined, removed solvent in vacuo producing the ethyl ester (27 g, 79%) as a yellow solid. This salicylaldehyde was of suitable purity to use without further purification. |
| 79% |
With triethylamine; magnesium chloride; In acetonitrile; at 10 - 72℃; for 2h; |
[0232] To a chilled solution of commercially available 2-iodophenol (30 g, 136 mmole) in ACN was added MgCl2 (19.5 g, 204 mmole) portion-wise while maintaining the temperature below 10 C, followed by PARAFORMALDEHYDE (28.6 g, 954 mmole) and TEA (76 mL, 545 mmole) producing a 15 C exotherm. The solution was heated to 72 C for 2 h. The reaction was cooled to room temperature and poured into Saturated aqueous Ammonium Chloride (500 mL), extracted with ethyl acetate (2 X 150 mL). The combined organic phases were washed with aqueous NAHC03 solution (2 X 150 mL), aqueous 1N HCL solution (2 X 150 mL), and brine (2 X 150 mL), dried OVER NA2S04, filtered and concentrated in vacuo. The crude material was subjected to flash chromatography (Silica, 5% Ethyl ACETATE/HEXANE). Desired fractions were collected and combined, removed solvent in vacuo producing the ethyl ester (27 g, 79%) as a yellow solid. This salicylaldehyde was of suitable purity to use without FURTHER PURIFICATION. HNMR (DMSO-D6/400 MHz) 10.95 (s, 1H), 10.19 (s, 1H), 7.33 (m, 3H), 4.31 (m, 1H). |
| 79% |
With triethylamine; magnesium chloride; In acetonitrile; at 10 - 72℃; for 2h; |
EXAMPLE 14; (2R)-6-chIoro-5-(3,3-dimethylbutyl)-2-(trifluoromethyl)-l,7b- dihydrocyclopropa[c]chromene-la(2H)-carboxylic acid; Step 1. Preparation of 2-hydroxy-4-iodobenzaldehyde.; [0185] To a chilled solution of commercially available 2-iodophenol (30 g, 136 mmole) in ACN was added MgCl2 (19.5 g, 204 mmole) portion-wise while maintaining the temperature below 10 0C, followed by paraformaldehyde (28.6 g, 954 mmole) and TEA (76 niL, 545 mmole) producing a 15 C exotherm. The solution was heated to 72 0C for 2 h. The reaction was cooled to room temperature and poured into Saturated aqueous Ammonium Chloride (500 mL), extracted with ethyl acetate (2 X 150 niL). The combined organic phases were washed with aqueous NaHCO3 solution (2 X 150 mL), aqueous IN HCl solution (2 X 150 mL), and brine (2 X 150 mL), dried over Na2SO4, filtered and concentrated in vacuo. The crude material was subjected to flash chromatography (Silica, 5% Ethyl acetate/ Hexane). Desired fractions were collected and combined, removed solvent in vacuo producing the ethyl ester (27 g, 79%) as a yellow solid. This salicylaldehyde was of suitable purity to use without further purification. 1HNMR (DMSO-J6/400 MHz) 10.95 (s, IH), 10.19 (s, IH), 7.33 (m, 3H), 4.31 (m, IH). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping